Key Takeaways (expand)
- Vegetable oils, often referred to as “seed oils” or “industrial seed oils”, include canola, corn, cottonseed, peanut, safflower, soybean, and sunflower oils.
- Vegetable oil myths generally focus on their high linoleic acid content and the processing methods used to extract them.
- Linoleic acid, an omega-6 fatty acid, is essential for human health, playing roles in immune response, cell signaling, and metabolic regulation.
- Linoleic acid is known for its cholesterol-lowering effects, which can help reduce the risk of heart disease.
- Studies show that linoleic acid does not significantly increase arachidonic acid levels nor inflammation in the body.
- Dihomogamma linolenic acid (DGLA), a beneficial omega-6 fat, is also produced from linoleic acid and has anti-inflammatory effects.
- Oxidation of linoleic acid, a common concern, is not a significant issue in the body when consumed in a normal diet.
- Human studies show that higher linoleic acid intake is associated with a lower risk of all-cause mortality, cardiovascular disease, cancer, diabetes, and stroke.
- The omega-6 to omega-3 ratio is less important than previously thought, as both types of fats can be protective against chronic diseases.
- Higher levels of both omega-6 (like linoleic acid) and omega-3 fats are linked to better health outcomes, as long as omega-3 fat intake is adequate.
- Historical studies that suggested negative effects of vegetable oils had significant limitations, including the use of trans fats in margarine and high participant dropout rates.
- Canola oil, a type of vegetable oil, has a favorable fatty acid profile, including high levels of monounsaturated fats and omega-3s, making it one of the healthiest cooking oils. Canola oil is also rich in phytosterols, which help lower LDL cholesterol and have anti-cancer, anti-inflammatory, and antibacterial properties.
- Despite concerns, the processing of vegetable oils, including the use of high heat and solvents like hexane, does not lead to significant health risks. Hexane residues in vegetable oils are minimal and well below safety thresholds, making these oils safe for consumption.
- The refining process of vegetable oils increases their stability, shelf life, and resistance to rancidity, contrary to some claims.
- Cooking with vegetable oils at moderate temperatures is safe, though they may not be the best choice for repeated high-temperature frying.
- Overall, the evidence supports the consumption of vegetable oils as part of a balanced diet, particularly when paired with adequate omega-3 intake.
Table of Contents[Hide][Show]
- Which Vegetable Oils Are We Talking About?
- Linoleic Acid: A Primer
- Linoleic Acid Research: Cell vs. Animal vs. Human
- Linoleic Acid, Arachidonic Acid, and Inflammation
- …Let’s Not Forget About Dihomogamma Linolenic Acid!
- What About Oxidation?
- Omega-6/Omega-3 Ratio: Important Balance, or Red Herring?
- A Note on FADS Genotype
- A Spotlight on Canola Oil
- What About the High Heat and Hexane?!
- Context and Cooking Method Matters, Too!
- Bottom Line
In the nutrition world, few topics are currently as polarizing as vegetable oils! While mainstream health guidelines typically advocate for vegetable oil consumption due to their favorable effects on LDL cholesterol levels, concerns have also circulated about their inflammatory potential, high omega-6 content, susceptibility to oxidizing, chemical solvents used for extraction and processing, and more. And on the surface, some seemingly compelling mechanisms and observational trends seem to support these fears.
However, a closer look at the research—particularly in humans—paints a different picture. In this article, we’ll be reviewing the full scope of evidence to see what the science really says!
The concerns surrounding vegetable oils generally boil down to two main arguments:
- problems associated with their high content of linoleic acid, and
- problems associated with the way they’re processed (namely, the use of high heat and chemical solvents such as hexane).
Let’s break down these arguments piece by piece!
Which Vegetable Oils Are We Talking About?
For starters, let’s define our terms!
While “vegetable oil” is somewhat of a misnomer when it comes to the specific oils under question, so too is the inflammatory language (hyuck, see what I did there?) used by health influencers and wellness gurus to perpetuate fear of vegetable oils (like calling them “industrial seed oils” or “industrially processed seed oils”). So what exactly is a vegetable oil? We’re talking specifically about oils that come from the endosperm (seed) of plants, and that are high in omega-6 polyunsaturated fats—namely linoleic acid. These include:
- Canola (rapeseed) oil
- Corn oil
- Cottonseed oil
- Peanut oil
- Safflower oil
- Soybean oil
- Sunflower oil
Note that this list doesn’t feature other plant oils such as coconut, avocado, palm, or olive oil—all of which have fatty acid profiles higher in saturated or monounsaturated fat, and as a consequence, a different array of health effects. Due to their higher omega-3 content, flaxseed and hemp seed oil are omitted here as well!
Linoleic Acid: A Primer
Linoleic acid is a polyunsaturated omega-6 fatty acid, and one of only two essential fatty acids for humans (the other being the omega-3 fatty acid alpha-linolenic acid). Linoleic acid plays a number of crucial roles in the body: for instance, various enzymes can oxidize linoleic acid to produce oxidized linoleic acid metabolites (also called OXLAMs), which are then involved in immune response, cell signaling, inflammation, and pain regulation. Linoleic acid can also bind to peroxisome proliferator-activated receptor alpha (or PPAR-α)—a transcription factor heavily involved in metabolic regulation. PPAR-α helps control the transport of fatty acids, inhibits fat formation (lipogenesis), and activates enzymes involved in fat breakdown and oxidation.
In nutrition science, linoleic acid’s major claim to fame is its potent cholesterol-lowering effects. Specifically, linoleic acid lowers LDL cholesterol by increasing bile acid production, enhancing cholesterol breakdown, upregulating the LDL receptor, moving LDL cholesterol out from the blood and into body tissues, and decreasing the conversion of very low density lipoprotein (VLDL) to LDL. For this reason, early research into linoleic acid (and subsequent nutrition recommendations) focused on its role in preventing heart disease.
So, why has this fat gotten a bad rap?
Here’s the plot twist! Along with its aforementioned functions, linoleic acid also serves as a precursor for all other omega-6 fats—including a very small amount (0.3 to 0.6%) getting converted to arachidonic acid. Arachidonic acid is an omega-6 fat that, in excess, has been implicated in a number of chronic diseases due to its inflammatory potential. So here’s the argument…
More specifically, arachidonic acid can be converted into bioactive lipid mediators called eicosanoids, including prostaglandins (which are synthesized in damaged tissue and help generate the inflammatory response), leukotrienes (which are involved in asthmatic and allergic reactions, and help sustain inflammatory responses in general), and thromboxane (which is released from blood platelets and causes them to aggregate—AKA clump together). Although these lipid mediators are important for normal metabolic function, their over-production has been linked to a higher risk of cancer and other inflammation-related conditions. So, the fact that linoleic acid can potentially increase the body’s arachidonic acid pool and lead to greater production of eicosanoids has sparked some concern!
On top of this, linoleic acid competes with omega-3 fats for the same conversion enzymes in the body. This is why we often hear about the importance of a balanced ratio between omega-6 and omega-3 fats: too much dietary omega-6 relative to omega-3 could interfere with omega-3 metabolism, potentially inhibiting their beneficial anti-inflammatory effects. (Spoiler alert: We also don’t need to worry about the ratio of omega-6s to omega-3s in our diets; more on that below!)
In addition to competition for enzymes, linoleic acid has been shown to decrease the incorporation of omega-3 fats into phospholipid membranes. In fact, some research suggests increased consumption of linoleic acid could be contributing to the lower concentrations of omega-3 fats observed in human tissue over time. In regions where vegetable oil intake is high and fatty fish intake is relatively low (such as the United States), the amount of linoleic acid in fat tissue has increased by an estimated 130%, with a simultaneous drop in the proportion of omega-3s. This has potential implications for cell signaling, gene expression, membrane protein function, and more.
As a polyunsaturated fat, linoleic acid is also more susceptible to oxidation than saturated and monounsaturated fats. This is due to the multiple (“poly”) double bonds in its molecular structure, which are less stable and more reactive to oxygen than single bonds. This has stirred up some fear that linoleic acid-rich vegetable oil is not only partly oxidized by the time we bring it home from the store, but that the linoleic acid we ingest makes cells in our bodies more prone to oxidizing, too—including our LDL particles. That spells trouble for heart disease.
Lastly, due to increased vegetable oil production over the last century, the average linoleic acid intake has risen from about 1 to 2% of calories prior to the late 1930s, up to over 7% of calories today. This dramatic increase seems to have occurred in tandem with chronic disease rates, implicating linoleic acid (and vegetable oils at large) as potential agents in everything from cardiovascular disease to diabetes to cancer. While correlation can’t prove causation here, this observational trend does seem alarming in the context of plausible mechanisms linking linoleic acid with inflammation and oxidation.
Whew! That looks like a lot of strikes against linoleic acid, huh? There’s just one problem… when we look at the research in humans, these fears simply don’t pan out!
Linoleic Acid Research: Cell vs. Animal vs. Human
The main evidence against vegetable oils comes from animal and in vitro research, which show specific, isolated mechanisms by which linoleic could cause harm. It’s easy to selectively choose from these studies to build a case against linoleic acid (in fact, the same could be done for nearly any nutrient!).
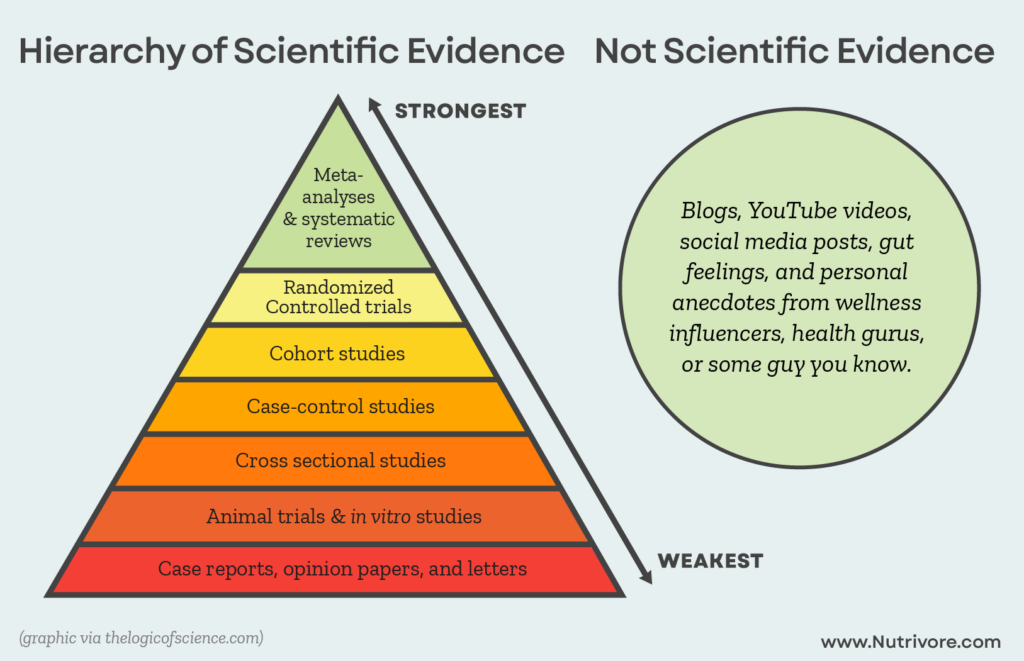
However, while these types of studies are useful for giving direction to better-quality research (and helping us understand the mechanisms behind observed phenomena), they’re categorically inferior to studies conducted in humans. Differences in physiology between animals and humans, as well as the controlled but artificial conditions of in vitro experiments, limit how applicable their findings are to the complex inner workings of the human body.
Where linoleic acid is concerned, what ultimately matters isn’t what happens in a mouse or test tube, but what happens in the human body after consumption. Luckily, we have plenty of research to shed light on that topic! (And as a result, no need to rely on lower-quality animal or in vitro research to make our claims.)
Linoleic Acid, Arachidonic Acid, and Inflammation
The evidence in humans overwhelmingly shows that linoleic acid consumption, as well as linoleic acid levels in the body, are either neutrally or inversely associated with inflammation. That’s good news!
Given that linoleic acid has the potential to be converted into arachidonic acid and subsequent inflammatory mediators, the next question is: does eating it actually do these things in humans? Let’s look at what the research here shows!
First of all, while the conversion pathway definitely exists, it’s not clear that linoleic acid intake (or vegetable oil intake in general) actually alters arachidonic acid levels in any meaningful way. For example, a 2011 systematic review of clinical trials found no impact of linoleic acid intake on arachidonic acid levels in the body, even when linoleic acid intake was decreased by as much as 90% or increased by as much as 600%. A randomized controlled trial from 2023 likewise found that among overweight or obese women, lowering linoleic acid intake had no independent effects on plasma arachidonic acid levels. And, a 2017 cross-sectional study found that higher intakes of polyunsaturated fat-rich cooking oils had no association with plasma arachidonic acid levels (red meat intake, however, did).
Second of all, even when it is produced, arachidonic acid hasn’t been consistently linked to pro-inflammatory effects or adverse health outcomes. A 2021 review of randomized controlled trials concluded that increasing arachidonic acid intake (up to 1500 mg/day) had no negative effects on markers of inflammation, immune function, platelet aggregation, or blood clotting, but that it might even benefit muscle and cognitive performance. A 2019 analysis of 30 prospective observational studies found that the highest versus lowest quintile of arachidonic acid levels (used as biomarkers for intake) were associated with an 8% lower risk of cardiovascular disease.
In addition, while some of the eicosanoids produced by arachidonic acid are inflammatory, others are anti-inflammatory (such as such as prostacyclin, lipoxin A4,11, and epoxyeicosatrienoic acids, say that five times fast!). So, even when small amounts of linoleic acid get converted into arachidonic acid, an inflammatory outcome isn’t guaranteed!
But, could linoleic acid be pro-inflammatory through any other avenues? Spoiler alert: it’s a “no” here, too! In fact, the evidence in humans overwhelmingly shows that linoleic acid consumption, as well as linoleic acid levels in the body, are either neutrally or inversely associated with inflammation. For example:
- A 2012 systematic review, encompassing 15 randomized controlled trials of healthy non-infant populations, investigated the effects of dietary linoleic acid on inflammatory biomarkers. None of the studies found any significant effects of linoleic acid on indicators of inflammation—including C-reactive protein, plasminogen activator inhibitor type 1, fibrinogen, soluble vascular adhesion molecules, cytokines, or tumor necrosis factor-α. The study authors stated, “We conclude that virtually no evidence is available from randomized, controlled intervention studies among healthy, noninfant human beings to show that addition of LA [linoleic acid] to the diet increases the concentration of inflammatory markers.”
- A 2003 study of healthy adults found that those with the highest intakes of both omega-6 and omega-3 fats had the lowest levels of inflammation. Overall, omega-6 intake didn’t inhibit the anti-inflammatory effects of omega-3 fats, but actually seemed to enhance those effects!
- A 2009 study of Japanese adults found that among men, intake of linoleic acid and alpha-linolenic acid were both significantly inversely associated with CRP concentrations—suggesting that each of these fats had beneficial effects on systemic inflammation. (In women, neither fat showed a significant relationship with CRP levels.)
In other words, there’s virtually no line of evidence in humans to suggest that linoleic acid increases inflammation. Instead, linoleic acid has either a neutral effect or it lowers inflammation!
…Let’s Not Forget About Dihomogamma Linolenic Acid!
The conversion pathway that produces arachidonic acid from linoleic acid also produces a highly beneficial (and less frequently discussed!) omega-6 fat: dihomogamma linolenic acid, or DGLA. Specifically, the enzyme delta-6-desaturase first converts linoleic acid into gamma linolenic acid (GLA), which can then undergo a two-carbon chain elongation by elongase to become DGLA.
DGLA has demonstrated a number of beneficial effects in the body. For example, it gets metabolized into anti-inflammatory signaling molecules such as prostaglandins of series 1 (PGE1) and thromboxane A1, which can enhance vasodilation (the widening of blood vessels), prevent platelet aggregation (AKA the clumping together of platelets in the blood), and reduce inflammation.
DGLA has also been shown to inhibit some critical processes involved in atherosclerosis—including monocyte migration, modified LDL uptake, foam cell formation, and inflammatory cytokine production. Similarly, DGLA metabolites can reduce blood pressure, enhance blood vessel dilation, and inhibit smooth muscle cell proliferation (a component of atherosclerotic plaque formation). Some research has even linked higher red blood cell levels of DGLA to significantly lower risk of stroke, heart attack, and death among people with existing cardiovascular disease (per a 2017 study, people with the highest versus lowest DGLA levels had a 43% lower risk of these three endpoints over the course of seven years!). Likewise, a 2021 clinical trial found that among elderly patients who recently had a heart attack, higher levels of DGLA in the blood were associated with a 46 – 53% lower risk of death over the course of the next two years.
Whether linoleic acid ultimately produces more arachidonic acid or DGLA is determined by a number of dietary, metabolic, and genetic factors. For one, zinc, magnesium, vitamin C, vitamin B3, and vitamin B6 all serve as cofactors for the enzymes involved in synthesizing DGLA from GLA, so being low in these nutrients can limit how much DGLA we can form. (In fact, the ratio of linoleic acid to DGLA in the blood is sometimes used to assess the presence of zinc deficiency!) Likewise, the omega-3 fat EPA has been shown to block delta-5-desaturase activity (the final enzymatic step in arachidonic acid synthesis), leading to higher blood levels of DGLA and lower levels of arachidonic acid and its inflammatory metabolites. As usual, omega-3s for the win! On top of that, genetic variations within the fatty acid desaturase (FADS) gene cluster can alter how much GLA gets converted into DGLA versus arachidonic acid, altering the ratio of these end-products by up to a three-fold difference.
So, while the potentially harmful conversion products of linoleic acid often steal the spotlight (needlessly so, as we’ve seen), this fat can also produce some very beneficial DGLA. And, whether it does so is largely a matter of eating a micronutrient-replete, omega-3 rich diet, a.k.a. Nutrivore, and with a dash of genetic variation thrown in!
Gamma Linolenic Acid
Gamma linolenic acid (GLA) is a polyunsaturated omega-6 fatty acid that may reduce symptoms of some chronic inflammatory diseases, particularly rheumatoid arthritis and atopic dermatitis. It may also benefit menstrual issues, diabetic neuropathy, cardiovascular health, and cancer—particularly via its conversion into other fatty acids and metabolites. Along with coming from food, it can be produced from another fat, linoleic acid.
What About Oxidation?
Even here, the research is inconsistent and requires some cherry-picking to ensure linoleic acid looks bad!
Another argument against linoleic acid involves its susceptibility to oxidation—not just going rancid in the bottle, but also increasing oxidation in our body due to its incorporation into cell membranes (which then become more vulnerable to free radical damage). This includes making our LDL particles more susceptible to oxidizing, which could play a role in the development of atherosclerosis. In addition, oxidized linoleic acid metabolites—called OXLAMs—have been associated with a number of diseases, adding even more fuel to the anti-linoleic-acid fire!
As with other arguments against this fat, we can find mechanistic studies that seem to confirm it. For example, an experiment from 1993 found that after feeding participants (in this case, people with mildly elevated cholesterol levels) diets high in either linoleic acid or monounsaturated oleic acid, LDL particles isolated from the linoleic acid group oxidized more quickly (as tested by exposure to copper, a strong oxidant). Several additional experiments from the early 1990s showed similar effects when linoleic acid and oleic acid were tested against each other.
However, even here, the research is inconsistent and requires some cherry-picking to ensure linoleic acid looks bad! While linoleic acid feeding creates more LDL-prone particles compared to oleic acid (again, in isolated controlled settings outside the body, and in a small number of available studies), its effects are actually similar to that of dietary saturated fat. A 1996 experiment included feeding participants different diets high in saturated fat (from butter and palm oil), high in long-chain omega-3 fats (from fatty fish), high in linoleic acid (from sunflower oil), and high in oleic acid (from olive oil), instead of testing only linoleic acid versus oleic acid. Lo and behold, this study found that diets high in saturated fat, linoleic acid, and omega-3 fats all produced LDL particles with similar resistance to oxidation; oleic acid still fared the best. (Interestingly, the high omega-3 diet also produced the greatest monocyte adhesion to endothelial cells, which typically suggests increased atherogenic risk. Given the overwhelming research linking long-chain omega-3s to better heart health, this is a great example of why these types of experimental studies aren’t always predictive of what happens in real-world settings!)
In fact, as more experiments of this type were conducted, the evidence began mounting that linoleic acid isn’t uniquely harmful among the fats, but that that oleic acid is uniquely beneficial!
What’s more, dietary fat modulation has a relatively small impact on LDL oxidation susceptibility relative to other dietary and lifestyle factors. Vitamin E intake, in particular, is a critical factor in how readily LDL particles oxidize—with even small (e.g., 25 mg per day) increases in vitamin E intake significantly reducing the susceptibility of LDL to oxidize. Although research here is extremely limited, some studies have tried to determine just how much our vitamin E requirements go up in order offset the increases in oxidation, with estimates landing around 0.6 mg of alpha-tocopherol per gram of dietary linoleic acid.
In addition, the body is a far more complex system than the controlled setting of these oxidation experiments. What ultimately matters is whether consuming more linoleic acid actually results in the disease outcomes that oxidized LDL could be linked to. As we’ll see shortly, that isn’t the case!
How Does Linoleic Acid Impact Disease Risk?
While research on linoleic acid intake and inflammation or oxidation is informative, there’s an even more important question to ask: How does linoleic acid consumption impact actual health and disease outcomes?
Fortunately, we have plenty of research here to draw from! Our best available studies overwhelmingly show that higher levels of linoleic acid (from the diet or in the body) are linked to:
- Lower risk of all-cause mortality
- Lower risk of death from cardiovascular disease
- Lower risk of death from cancer
- Lower risk of diabetes
- Lower risk of stroke
- Lower risk of coronary heart disease events
Yes, that’s right. The truth is actually the opposite of the anti-seed-oil propaganda! Vegetable oils are actually beneficial for our health!
Here’s a more detailed rundown of the findings from meta-analyses, which provide the highest-quality evidence possible on this topic (due to synthesizing a large body of research, increasing statistical power, reducing bias, and showing us where the preponderance of evidence lies!).
Vegetable Oil Reduces All-Cause Mortality and Disease-Related Deaths
A 2020 systematic review and meta-analysis, encompassing 44 prospective cohorts and a total of over 800,000 participants, assessed the relationship between dietary intake and biomarkers of linoleic acid and subsequent mortality outcomes. The results showed a significant protective effect of linoleic acid against all-cause mortality and against disease-related deaths. Specifically, participants consuming the highest versus lowest amounts of linoleic acid had a 13% lower risk of death from all causes, 13% lower risk of cardiovascular disease mortality, and 11% lower risk of cancer mortality!
Similarly, each standard deviation increase of linoleic acid concentration in the body (adipose tissue or blood) was associated with better mortality outcomes, including a 9% lower risk of total mortality, a 11% lower risk of cardiovascular disease mortality, and a 9% lower risk of cancer mortality.
Vegetable Oil Reduces Cardiovascular Disease Risk
Along with being protective against cardiovascular-related deaths, linoleic acid appears protective against cardiovascular events! A 2014 systematic review and meta-analysis of prospective cohort studies, encompassing over 310,000 participants, found that the highest versus lowest category of linoleic acid intake was associated with a 15% lower risk of coronary heart disease events (on top of a 21% lower risk of coronary heart disease deaths). And for stroke? A 2020 systematic review and meta-analysis of 11 observational studies, including a total of 47,836 participants, found that higher dietary linoleic acid intake was associated with a 13% lower risk of hemorrhagic stroke.
Vegetable Oil Reduces Type 2 Diabetes Risk
And what about linoleic acid intake and type 2 diabetes—another chronic disease that seems to have risen at the same time vegetable oil intake increased? A 2021 systematic review and dose-response meta-analysis offers some insight here! Encompassing 31 prospective cohorts and over 380,000 participants, this analysis found that higher (versus lower) linoleic acid intake was associated with a 6% reduction in type 2 diabetes risk. What’s more, every 5% increase in energy from linoleic acid intake was associated with a 10% drop in diabetes risk. And for every standard deviation increment in linoleic acid concentrations in adipose tissue or blood, type 2 diabetes risk decreased by 15%. A 2017 pooled analysis of individual-level data from 20 prospective cohort studies, including a total of 39,740 adults, similarly found higher proportions of linoleic acid biomarkers as percentages of total fatty acid were associated with a lower risk of type 2 diabetes.
Vegetable Oil Reduces Breast Cancer Risk
Lastly, while findings for linoleic acid and specific cancer risks haven’t been consistent, breast cancer may be an exception. A 2016 meta-analysis of eight prospective cohort studies, including a total of 358,955 adult women, found that breast cancer risk decreased by 1% for every 10 gram per day increase in linoleic acid intake. Small but non-statistically-significant protective associations were also found between the highest and lowest levels of dietary and serum linoleic acid.
Collectively, these findings are consistent with the idea that long-term linoleic acid intake does not increase chronic disease or mortality risk, but may even be protective!
Omega-6/Omega-3 Ratio: Important Balance, or Red Herring?
The common narrative that “omega-3 = good, omega-6 = bad” is not only oversimplified, it’s actually fundamentally wrong.
Apart from the specific effects of linoleic acid, another argument against omega-6-rich vegetable oils is their ability to drive up the overall omega-6/omega-3 ratio of the diet. The concern here involves the competition between these two fats in the body: linoleic acid and alpha-linolenic acid require the same enzymes (delta-6-desaturase and delta-5-desaturase) for conversion into their long-chain forms (arachidonic acid and EPA/DHA, respectively), and also compete for placement in cell membranes. So, in theory, excessive intake of omega-6 fats could interfere with the body’s use of omega-3s.
Adding to this argument is the fact that our modern diets feature a vastly skewed omega-6/omega-3 ratio compared to historical levels. For most of our past, the human diet had an estimated 1:1 ratio of omega-6 to omega-3 fats, whereas the modern Western diet has a ratio of 15 to 20:1 or higher!
So, it seems reasonable to suspect that vegetable oils could be harming us due to their excessive contribution of omega-6 fats relative to our omega-3 intake. Once again, though, we must ask: what does the research in humans show?
It turns out, the evidence for the omega-6/omega-3 ratio mattering in human health is much less compelling than once thought! In fact, many studies show that higher levels of linoleic acid and omega-3 fatty acids are both protective against all-cause mortality, disease-specific mortality, and some chronic health conditions; generally, omega-3s are just the more protective of the two. This suggests that as long as omega-3 levels are adequate, higher omega-6 intake (and the ratio it creates with omega-3 fats) may be of lesser concern, if not beneficial.
For example, a 2015 population-based cohort of 4,232 Swedish older adults found that serum levels of linoleic acid and very-long-chain omega-3 fats (partly reflective of vegetable oil and fish oil intake, respectively) were all independently, inversely associated with total mortality. In fact, linoleic acid was slightly more protective—27% lower risk for higher versus lower levels, compared to 19% for EPA and 20% for DHA!
A 2021 analysis of National Health and Nutrition Examination Survey (NHANES) data, tracking 4,132 participants with a mean follow-up of almost seven years, found that serum levels of linoleic acid and individual omega-3 fats (alpha-linolenic acid, EPA, and DHA) were all associated with significant reductions in all-cause mortality. Again, this supports the idea that while more omega-3 fats are great, it doesn’t need to be at the expense of lowering omega-6 intake!
A 2014 multicenter study of 2,792 participants free from cardiovascular disease at baseline found that higher circulating levels of linoleic acid were significantly protective of both total mortality and cardiovascular disease mortality. In a follow-up comment, the authors noted finding little evidence of any interaction between linoleic acid and omega-3 fats—but rather, that the benefit of linoleic acid was additive, with participants having the greatest risk reduction when both their linoleic acid levels and omega-3 levels were highest.
A 2012 cross-sectional study of 2,451 adults found that intake of omega-3 fats (as alpha-linolenic acid) and total omega-6 fats were both inversely associated with metabolic syndrome; in fact, participants in the highest versus lowest quartile of omega-6 intake had a 47% lower prevalence of metabolic syndrome. What’s more, participants consuming at least the median intake of alpha-linolenic acid (1084 mg per day) had lower rates of metabolic syndrome regardless of what their omega-6 intake was. In other words, omega-6 consumption had no impact on metabolic syndrome risk as long as omega-3 intake was high enough, and the ratio of omega-6 to omega-3 had no effect on metabolic syndrome risk!
A 2013 analysis of the Multi-Ethnic Study of Atherosclerosis, encompassing 2,837 adults, found that higher levels of EPA and DHA (both from the diet and in the blood) were associated with lower cardiovascular disease incidence, while omega-6 fats (linoleic acid and arachidonic acid) bore no relationship to cardiovascular disease risk. Interestingly, alpha-linolenic acid was also unassociated with cardiovascular disease risk in this study. This supports the idea that long-chain omega-3 fats are independently beneficial, regardless of omega-6 status.
And, here’s a great example of why reading the actual study (and not just the headline) is important! A 2024 population-based cohort study, encompassing more than 85,000 participants from the UK Biobank, was published with the title “Higher ratio of plasma omega-6/omega-3 fatty acids is associated with greater risk of all-cause, cancer, and cardiovascular mortality.” While this finding was technically true, there’s much more to the story! When these fats were assessed independently (rather than as a ratio), it turned out that high levels of omega-6 fats and omega-3 fats were both associated with a lower risk of death; omega-3 fats were just more strongly protective. Specifically, participants with the highest omega-6 levels had a 23% lower risk of dying from any cause, while those with the highest omega-3 levels had a 31% lower risk. The greater protection offered by omega-3s explains why a high omega-6 to omega-3 ratio was linked to harm, even though both fats were beneficial.
It’s also worth noting that while omega-3 fats typically demonstrate superior benefits compared to omega-6 fats, this isn’t always the case! Some studies have found that when both omega-6 and omega-3 fats are evaluated, omega-6 fats end up being the more protective of the two.
For example, a 2016 observational study of patients with acute decompensated heart failure found that the over the course of follow-up (a median of 560 days), those who had higher red blood cells levels of omega-6 fats were significantly less likely to experience either a worsening of their heart failure, or death from any cause. Meanwhile, red blood cell levels of omega-3 offered no protection against the risk of these adverse events.
Likewise, a 2022 study tracked the health outcomes of 8,537 patients with cardiometabolic disease (including cardiovascular disease and type 2 diabetes) for a median follow-up time of 10.3 years. The results showed that those in the highest versus lowest tertile of linoleic acid intake had a 14% lower risk of death from all causes. Interestingly, this association was non-linear, with the risk reduction plateauing when linoleic acid was 7.5% of total energy. Meanwhile, consumption of the omega-3 fats EPA and DHA had no significant protective effect here!
In other words, the common narrative that “omega-3 = good, omega-6 = bad” is not only oversimplified, it’s actually fundamentally wrong. A better way to look at it is this: For the majority of health outcomes, omega-6 fats range from neutral to beneficial, while omega-3 fats range from beneficial to extremely beneficial! Nowhere does the science support the claim that omega-6 fats are innately, independently harmful; nor does their ratio with omega-3 fats appear to be important, once absolute intakes of both fats are accounted for.
All that being said, even if we found evidence that the omega-6/omega-3 ratio is an important driver of disease risk, there’s an easy fix that doesn’t involve avoiding vegetable oils: eating more omega-3 rich foods! Yep, it’s really that simple. (And excellent advice in general!)
Older Long-Term Trials: A Smoking Gun?
In the 1960s through 1970s, various long-term trials were conducted to investigate the validity of the Diet-Heart Hypothesis (AKA the idea that saturated fat consumption raises heart disease risk), which had gained scientific interest at the time. Most of these studies involved replacing saturated fats with polyunsaturated fats, and then tracking participants’ disease and/or mortality outcomes during the subsequent years. Although generally lower-quality than more contemporary controlled trials, they were unique in the fact that their follow-up periods were so long—allowing for insight into the long-term effects of dietary changes, which are harder to capture in shorter-running studies.
Three of these trails, in particular, have been cited as evidence that vegetable oils negatively affect health over time: the Sydney Diet Heart Study, the Minnesota Coronary Experiment, and the LA Veterans Administration Study. Collectively, these studies seem to suggest that replacing saturated fats with vegetable oils could increase total mortality, heart disease mortality, and/or cancer incidence.
However, these studies have a lot of moving parts that affect how we should interpret them! Let’s take a look at these famous trials and what they found.
The Sydney Diet Heart Study
The Sydney Diet Heart Study was a randomized controlled trial that ran from 1966 to 1973, designed to assess the effects of substituting saturated fats with safflower oil on cardiovascular and all-cause mortality. The study included 458 men aged 30 to 59 who had recently suffered a coronary event. Although it was originally published in 1978, an updated analysis of the data in 2013 brought the study back into the spotlight—seemingly implicating vegetable oils in higher risk of death from all causes, coronary heart disease, and cardiovascular disease. (The use of safflower oil was major strength of this study, since its polyunsaturated fat content is almost exclusively linoleic acid—preventing any confounding from simultaneously raising omega-3 intake!)
While the original publication only reported total mortality, the 2013 re-analysis further included deaths from coronary heart disease and cardiovascular disease. Between the two publications, the study showed that compared to the control group, the intervention (safflower oil) group had:
- A 62% higher incidence of total mortality
- A 70% higher incidence of coronary heart disease mortality
- A 74% higher incidence of cardiovascular disease mortality
Pretty scary, right?! Although these figures look like strong evidence against high-linoleic acid vegetable oils, an important detail casts doubt on this conclusion. The intervention group was given safflower oil not only in standard liquid form, but also as a soft margarine—specifically, a brand called Miracle. At the time the study was conducted, Miracle margarine contained about 15% trans fatty acids, likely causing a significant increase in the participants trans fat intake. Although the harms of trans fats were unknown at the time, we now have abundant evidence that they’re amongst the most damaging fats for cardiovascular health—plausibly contributing to the intervention group’s increased mortality rates.
So, rather than straightforwardly testing the effects of replacing saturated fat with linoleic acid, the Sydney Diet Heart Study was likely testing the effects of increasing trans fat intake as well!
The Minnesota Coronary Experiment
The Minnesota Coronary Experiment is another randomized controlled trial testing the effects of replacing saturated fat with linoleic acid-rich vegetable oil (more specifically, corn oil). Conducted from 1968 to 1973, the trial included over 9,000 patients given either a control diet (18% saturated fat and 5% polyunsaturated fat) or an intervention diet (9% saturated fat and 15% polyunsaturated fat).
Results from this study were first published in 1989 and showed that while the vegetable oil intervention successfully lowered participants’ cholesterol levels (from an average of 207 mg/dL to 175 mg/dL), there was no significant difference in cardiovascular events, cardiovascular deaths, or total mortality when compared to the control group.
But, as with the Sydney Diet Heart Study, the Minnesota Coronary Experiment underwent a re-analysis in 2016 after the recovery of previously unpublished data. This new analysis showed that for each 30 mg/dL reduction in cholesterol levels, risk of death rose by 22%—although this trend was mostly limited to participants above the age of 65.
So, is this evidence that reducing cholesterol levels by increasing vegetable oil intake leads to a higher risk of death? Not so fast! This study had some extremely important limitations that make its results widely questioned:
- A staggering 83% of the participants were lost to follow up—meaning they were initially participating in the trial, but at some point became unreachable. This introduces the potential for attrition bias, AKA the selective dropout of participants who have important differences from those who stay in the study (including being “lost” because they became ill or died!).
- The 21 excess deaths in the vegetable oil group were exclusively among patients above the age of 65. In the control group, 15 more patients in this age range were lost to follow-up without explanation compared to the vegetable oil group. This calls into question whether those control group participants had also died, thereby skewing the study’s mortality findings.
- The association between greater reductions in cholesterol and higher risk of mortality wasn’t necessarily due to diet: some illnesses can also cause cholesterol levels to fall dramatically prior to death. Given that the study’s cholesterol/mortality finding was limited to participants older than 65 (a group already at higher risk of chronic disease), it’s plausible that declining cholesterol levels were an effect of an existing terminal illness, rather than a cause.
- As with the Sydney Diet Heart Study, participants in the Minnesota Coronary Experiment consumed some of their vegetable oil in the form of margarine, contributing an unknown amount of trans fat to their diets.
- The study also found that smoking, a higher body mass index, and higher diastolic blood pressure were all associated with a lower mortality risk. These findings run counter to the vast majority of higher-quality evidence, and shed more doubt on how accurate the study’s findings were!
So, between the extremely high loss of participants to follow-up, unknown trans fat intake, potential reverse causality for a cholesterol-mortality link, and anomalous findings of factors protective against mortality, we should think twice about counting this study as robust evidence against vegetable oils!
The LA Veterans Administration Study
The LA Veterans Administration Study was a randomized controlled trial of 846 male veterans, designed to test the substitution of vegetable oils for animal fats on the risk of cardiovascular disease. Both groups received diets containing about 40% of calories as fat—10% of that being linoleic acid in the control group, and 38% of that being linoleic acid in the intervention group. Participants were followed for up to eight years, and the study’s results were published in 1969.
When cardiovascular events were pooled together (including heart attacks, sudden death, and stroke), the vegetable oil group saw significantly fewer compared to the control group—an incidence rate of 31.3% versus 47.7%, respectively. The greatest benefits of increased vegetable oil consumption were seen for participants under 65.5 years old, as well as for those who had cholesterol levels above 233 mg/dL at baseline.
However, there was a surprising non-cardiovascular finding as well: the vegetable oil group experienced an excess of cancer deaths compared to the control group (31 deaths versus 17). What’s more, the control group contained more heavy smokers than the vegetable oil group (70 versus 45 participants smoking at least a pack a day)—a point often used to claim that the control group should have been at a higher risk of cancer to begin with, making the extra cancer deaths in the vegetable group even more concerning. As a result, this study is sometimes used as evidence that long-term vegetable oil consumption can increase the risk of cancer!
Once again, though, there’s more to the story. For one, the increased cancer deaths in the vegetable oil group didn’t reach statistical significance—meaning they can’t be ruled out as random chance. And even more importantly, many of the cancer deaths in the vegetable oil group were among the participants with the poorest adherence to the diet. In fact, when the study authors performed an analysis on the data to adjust for differences in diet adherence, they found that the cancer deaths were consistent with random distribution.
In other words, participants in the intervention group who were actually consuming more vegetable oils were not suffering from significantly higher cancer mortality. If vegetable oils were actually driving these deaths, we’d expect to see the opposite: more deaths among the better adherers, and fewer deaths among the poorer ones.
And as for the disproportionate number of smokers in the control group? Citing only the number of heavy smokers is misleading here! For one, 58 participants in the control group and 41 in the experimental group lacked any data on smoking habits, so we don’t know for sure how many heavy smokers were in each group. And for participants that did have smoking data, the vegetable oil group actually had slightly more total smokers than the control group (283 versus 279, respectively).
But Wait, There’s More!
Although the previous three studies are the most commonly cited in the anti-vegetable-oil narrative, they weren’t the only diet-heart trials of their era. In fact, the reason they’re so often referenced is because they’re the ones that showed unfavorable outcomes for the intervention group! Cherry-picking, much? Other studies conducted during this time period were more consistent with a neutral or beneficial effect of vegetable oils, including:
- The Oslo Diet-Heart Study, which tested the effects of consuming 15 to 30 grams per day of soybean oil (along with advice to eat more fish and less fat) on men with a prior history of heart attack. The intervention group saw a non-statistically-significant 43% reduction in fatal heart attacks compared to the control group.
- The Norwegian Vegetable Oil Experiment of 1965-1966, which found no difference in cardiovascular outcomes when 13,000 men were given flaxseed oil (high in alpha-linolenic acid) versus sunflower oil (high in linoleic acid) for a year.
- The Finnish Mental Hospital Study, which found a significant reduction in heart attack deaths among male patients eating a diet high in vegetable oils and low in meat and dairy fat.
However, as with the other trials of this era, these studies had important limitations and methodological flaws (for example, the Finnish Mental Hospital Study wasn’t technically a randomized controlled trial, and may have been confounded by the use of thioridazine—a medication that’s been linked to sudden death and heart abnormalities). So, while these studies were important for their time, many decades’ worth of higher-quality research has accumulated in the time since they were published.
Calm Your Food Fears by Busting 12 Common Myths

The Truth About Food Toxins
Eat healthier without fear, stress or breaking the bank.
Move past food fears with this detailed analysis of the science that busts the 12 most common myths about food “toxins.” This webinar includes an 80-minute video presentation and a 32-page written webinar summary with additional details and scientific citations.
Buy now for instant digital access.
A Note on FADS Genotype
While the polyunsaturated fat levels in our bodies are strongly influenced by diet, there’s another component here that deserves attention: genetics!
Specifically, there’s considerable genetic variation in the activity of the enzymes that convert short-chain omega fats (linoleic acid or alpha-linolenic acid) into their long-chain forms (arachidonic acid or EPA/DHA, respectively). These enzymes are encoded by two genes, FADS1 and FADS2, located on chromosome 11 in humans. Within these genes, a number of variants (called single nucleotide polymorphisms, or SNPs) exist, significantly impacting the activity and efficiency of these enzymes. As a result, some people have genetically enhanced conversion of linoleic acid into arachidonic acid, and of alpha-linoleic acid into EPA and DHA; meanwhile, other people have less efficient conversion.
Scientists believe these variants emerged due to selective pressure to adapt to agricultural diets higher in plant-based omega fats (e.g., from seeds and grains) and lower in animal-based or marine fats (such from as seafood). Supporting this hypothesis is the fact that FADS genotype has strong associations with ethnicity. For example, about 80% of people of African descent carry two copies of a major FADS SNP associated with higher arachidonic levels, compared to 46% of those with European ancestry, and virtually no one of Native American ancestry.
Along with influencing the conversion of the short-chain omega fats we consume, FADS variants have been shown to affect a number of risk markers for disease, as well as sometimes correlating with actual disease incidence. For example, the same SNPs that are associated with higher arachidonic acid levels are also associated with greater eicosanoid (inflammatory mediator) production, prostaglandin excretion (a marker for heart disease risk), LDL cholesterol, triglycerides, total cholesterol levels, C-reactive protein, and coronary artery disease risk.
Adding one more variable into the mix, some evidence suggests that genetic differences in the FADS gene cluster have stronger effects on omega-6 fats than omega-3 fats. For example, certain variants are associated with higher levels of arachidonic acid, but show no association with EPA and DHA—suggesting that the conversion of linoleic acid is more heavily impacted by these variants than alpha-linoleic acid. However, these findings could also be due to individual differences in omega-3 fat intake, which has more variation than omega-6 intake in Western diets. Due to limitations in the studies we have so far, we can’t tell for sure one way or another. Clearly, much more research is needed!
So, all this brings us to the question: could FADS variants impact how our bodies respond to eating linoleic acid? There’s some evidence for a “yes” here, but it comes with caveats!
A small number of human trials have been conducted to test the effects of linoleic acid consumption on people with different FADS variants. Although the results haven’t been totally consistent, some of them found a small but statistically significant increase in inflammation (as measured by C-reactive protein) for people with FADS variants associated with higher enzyme activity, but not for people with lower enzyme activity. While intriguing, the results weren’t generally dramatic enough to be clinically significant. Again, more research, with larger sample sizes, is needed to explore this topic!
What we can say for sure is that regardless of FADS genotypes, consuming more omega-3 fats (especially long-chain DHA and EPA) is protective. Even for individuals with genetically greater desaturase enzyme activity, the magnitude of any impact on inflammation appears small enough to be offset by other dietary components.
A Spotlight on Canola Oil

While canola oil gets lumped under the general “vegetable oil” umbrella (and therefore subject to the same critiques as other oils here), it actually has some properties that make it highly unique! In particular, its impressive fatty acid profile and high content of phytosterols make it one of the healthier plant oil options out there.
Canola oil traces its roots to an ancient crop from the Brassica genus (yep, it’s technically related to famous cruciferous vegetables like broccoli, cauliflower, cabbage, kale, and more!). Its “parent” plant, rapeseed, has been cultivated in India for at least 4000 years, and in China and Japan for 2000 years. Canola oil was bred from two rapeseed cultivars (Brassica napus and Brassica rapa) in the early 1970s, to produce a variety with seeds low in erucic acid (a monounsaturated fat that can be harmful at very high intakes) and bitter glucosinolates. The word “canola” comes from a combination of “Canada” (where this oil was first developed) and “ola,” based on the Latin word for oil.
One of canola oil’s standout features is its fatty acid profile. Unlike other vegetable oils, canola oil has relatively lower levels of linoleic acid (about 20% of total fat), and instead contains a predominance of monounsaturated fat (up to 66% of total fat). Nearly all of this comes from oleic acid—the same fat abundant in olive oil. In fact, in its upper range, the amount of oleic acid in canola oil is comparable to that in olive oil (which contains about 60 – 80% oleic acid, depending on the olive cultivar)!
Canola oil is also unusually high in the omega-3 fat alpha-linolenic acid, which comprises about 7 – 15% of its total fat composition (depending on the canola oil source and analysis). This makes it one of the highest omega-3 cooking oils out there!
Among the common cooking oils, canola oil has one of the highest contents of sterols—naturally occurring compounds found in the cell membranes of plants, which have a structure similar to that of cholesterol (which allows them to block the absorption of dietary cholesterol in the intestines). Although sterols are most famous for selectively lowering LDL cholesterol levels, they also exhibit anti-cancer, anti-inflammatory, antibacterial, and antifungal activities!
A 2019 analysis looked at the phytosterol content of 13 different oils: rapeseed (canola) oil, peanut oil, soybean oil, sesame oil, olive oil, camellia oil, corn oil, sunflower oil, flaxseed oil, rice bran oil, walnut oil, peony oil, and grapeseed oil. Of these, canola oil contained the third highest quantity of phytosterols (893 mg per 100 grams of oil), behind only rice bran oil and corn oil. (By comparison, this analysis found that olive oil contained 288 mg of phytosterols per 100 grams of oil.)
The phytosterol content of canola oil included 137 mg of brassicasterol, which demonstrates anti-cancer activity against liver cancer and bladder cancer, antiviral properties against hepatitis B and herpes simplex virus type 1 (HSV-1), antifungal activity against Mycobacterium tuberculosis (the bacteria responsible for tuberculosis infection), and angiotensin-converting enzyme (ACE) inhibiting activity (giving it a role in reducing hypertension)—and there’s still plenty more research to be done! Canola oil contains a number of other phytosterols as well, including 267 mg of campestanol, 394 mg of β-sitosterol, 41 mg of delta-5-avenasterol, 25 mg of stigmasterol, and smaller amounts of cycloartenol, methylene-cycloartanol, and egrosterol,
In controlled trials that compare the two side-by-side, canola oil sometimes demonstrates even more significant health benefits than olive oil—arguably the most uncontroversial oil out there! For example, a 2021 randomized controlled trial found that in women with PCOS, 10 weeks of canola oil consumption (25 grams daily) led to greater improvements than olive oil (as well as sunflower oil) when it came to lipid profiles and and insulin resistance (as measured by HOMA-IR). Specifically, canola oil had the biggest effect on reducing triglycerides, the total cholesterol/HDL ratio, and the triglyceride/HDL ratio. (Both canola oil and olive oil also reduced the severity of non-alcoholic fatty liver disease among the participants).
A 2020 systematic review and meta-analysis of 42 controlled clinical trials found that compared to other edible oils (including olive oil, saturated fats, and sunflower oil), canola oil consumption led to the greatest improvements in cardiometabolic risk factors. This included significantly reducing total cholesterol, LDL cholesterol, the LDL/HDL ratio, the total cholesterol/HDL ratio, apolipoprotein B (Apo B) levels, and the Apo B/Apo A-1 ratio. A dose-response analysis of the data showed that optimal effects on blood lipids occurred when consuming 15% of total calories as canola oil (specifically, when these calories displaced other dietary oils).
All this isn’t to say olive oil is bad; just that canola oil holds its own against well-established healthy foods! So, when it comes to vegetable oils, canola might deserve an extra special place at the table.
What About the High Heat and Hexane?!

Another argument against vegetable oils is the way they’re processed, which typically involves a mixture of high heat and chemical solvents. The proposed risk is twofold: fatty acid oxidation from the heat, and potentially toxic compounds remaining in the oil after extraction.
Although both of these points sound scary, they don’t hold up to scrutiny!
For one, while high temperatures may indeed used during extraction, it’s also done under high pressure—which means no oxygen (and therefore, no potential for oxidation)! Not only that, but lipid peroxidation is measured as part of the quality control for all oils that are on the store shelves. In other words, if you’re purchasing a commercially prepared oil, regulations are in place to ensure you’re not carrying home already-oxidized oil.
As far as toxic compounds involved in processing? Here’s the full scoop on how vegetable oils are made, and why the fear here is unfounded, too!
To make vegetable oil from plants, first the plant parts that are used to make the oil are harvested, deshelled, hulled, and cleaned. If the seed is used to make the oil, it is crushed or broken up into smaller pieces. Sometimes a first-press oil is made by mechanical extraction, basically squeezing these tiny pieces until oils comes out (a.k.a. cold pressing), and then a second-press oil is made from solvent extraction.
During solvent extraction, hexane is added to the crushed plant parts and then briefly heated, which causes a chemical reaction to allow the oil from the plant to separate out. The heat also causes hexane to evaporate—most vegetable oils have no detectable hexane residues. In one study, the highest concentration of hexane detected was 0.4 milligrams per kilogram (1.1 liters) of oil. The provisional chronic reference dose of hexane is 0.06 milligrams per kilogram bodyweight per day—so, a 70-kg person (154 pounds) would have to consume over 11 liters of this highest-hexane-detected vegetable oil every day to ingest enough hexane to worry! That would add up to about 97,000 calories worth of oil every day, not recommendable for a multitude of reasons, least of which is hexane! (By the way, hexane is used in the production of a number of other foods and supplements, including collagen powder!)
Once the oil is extracted, it’s then bleached using bleaching clay—not the bleach you use to clean—to remove any coloring pigments like carotenoids or chlorophyll which can make the oil more susceptible to oxidation in response to light. It’s then deodorized to remove any odor and off-flavors, and contaminants (pesticides, polycyclic aromatic hydrocarbons, etc.). Finally, it is filtered to remove any residual solvents, leaving the oil in its final state, which is packaged and sold. The refining process gives vegetable oil its light color, makes it flavorless and odorless, and makes it more stable with a longer shelf life. The claim that refining vegetable oil makes it more likely to go rancid is untrue. In fact, refining vegetable oil reduces the likelihood of rancidity!
In addition, expeller-pressed (cold-pressed) options for most vegetable oils exist and are easy to find at the grocery store, so you have plenty of options if you want to avoid refined vegetable oils.
Context and Cooking Method Matters, Too!
The form in which vegetable oils are consumed may play a role in their health effects. For example, some studies show that linoleic acid-rich oils used for deep frying have stronger inflammatory potential than similar oils that aren’t repeatedly exposed to high temperatures (such as in salad dressings), due to differences in oxidation. A 2014 prospective study showed that Americans who consume deep fried foods daily have a 21% increased risk for coronary heart disease compared to people who eat fried foods less than once per week. However, how much of this increased risk is due to repeatedly heating vegetable oils, too-high dietary fat in general, or other factors remains unknown. Notably, the people who ate the most fried foods in this study also had the overall lowest quality diets, the lowest vegetable and fruit consumption, the highest sugar-sweetened beverage consumption, and they had the lowest physical activity and were the most likely to smoke. The lower diet quality in the high-fried foods group is especially relevant since animal studies have also shown that linoleic acid produces significantly more harmful oxidative metabolites when consumed in the absence of vitamin E (an important fat-soluble antioxidant), indicating the importance of stabilizing oxidation-prone fats like linoleic acid with other dietary components. While sophisticated statistical analyses try to take all of these into account, it’s possible that healthy user bias explains these results, meaning that the people who ate less fast food had a constellation of other healthy behaviors that we can’t fully account for in the mathematical modeling. While this question remains unanswered in the scientific literature, it’s best to consume deep fried foods in moderation.
On the whole, evidence suggests that vegetable oils aren’t the best choice for repeated, high-temperature frying (there may not be a good choice for this culinary use), but cooking with them for a single use below their smoke point is perfectly safe!
Bottom Line
While we can build a case against vegetable oils from a mechanistic perspective, it just doesn’t hold up within living, real-world human bodies. The evidence is far more compelling in favor of consuming adequate omega-3-rich foods than keeping our vegetable oil intake low—especially in the context of a diverse, nutrient-dense diet!
Citations
Expand to see all scientific references for this article.
Amiri M, Raeisi-Dehkordi H, Sarrafzadegan N, Forbes SC, Salehi-Abargouei A. The effects of Canola oil on cardiovascular risk factors: A systematic review and meta-analysis with dose-response analysis of controlled clinical trials. Nutr Metab Cardiovasc Dis. 2020 Nov 27;30(12):2133-2145. doi: 10.1016/j.numecd.2020.06.007.
Anton SD, Heekin K, Simkins C, Acosta A. Differential effects of adulterated versus unadulterated forms of linoleic acid on cardiovascular health. J Integr Med. 2013 Jan;11(1):2-10. doi: 10.3736/jintegrmed2013002.
Aşkın B, Kaya Y. Effect of deep frying process on the quality of the refined oleic/linoleic sunflower seed oil and olive oil. J Food Sci Technol. 2020 Dec;57(12):4716-4725. doi: 10.1007/s13197-020-04655-4.
Barham JB, Edens MB, Fonteh AN, Johnson MM, Easter L, Chilton FH. Addition of eicosapentaenoic acid to gamma-linolenic acid-supplemented diets prevents serum arachidonic acid accumulation in humans. J Nutr. 2000 Aug;130(8):1925-31. doi: 10.1093/jn/130.8.1925.
Batool N, Arshad M, Hassan F, Ilyas N, Shahzad A. Report-Physicochemical and Antimicrobial properties of canola (Brassica napus L.) seed oil. Pak J Pharm Sci. 2018 Sep;31(5):2005-2009.
Bersch-Ferreira ÂC, Sampaio GR, Gehringer MO, Ross-Fernandes MB, Kovacs C, Alves R, Pereira JL, Magnoni CD, Weber B, Rogero MM. Association between polyunsaturated fatty acids and inflammatory markers in patients in secondary prevention of cardiovascular disease. Nutrition. 2017 May;37:30-36. doi: 10.1016/j.nut.2016.12.006.
Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr. 2011 May;93(5):950-62. doi: 10.3945/ajcn.110.006643.
Brown JB. Changes in nutritive value of food fats during processing and cooking. Nutr Rev. 1959 Nov;17:321-5. doi: 10.1111/j.1753-4887.1959.tb03553.x.
Cahill LE, Pan A, Chiuve SE, Sun Q, Willett WC, Hu FB, Rimm EB. Fried-food consumption and risk of type 2 diabetes and coronary artery disease: a prospective study in 2 cohorts of US women and men. Am J Clin Nutr. 2014 Aug;100(2):667-75. doi: 10.3945/ajcn.114.084129.
Choque B, Catheline D, Rioux V, Legrand P. Linoleic acid: between doubts and certainties. Biochimie. 2014 Jan;96:14-21. doi: 10.1016/j.biochi.2013.07.012.
Clifton P. Comment on Ramsden et al. Br J Nutr. 2011 Sep;106(6):958; author reply 959-60. doi: 10.1017/S0007114511004466. PMID: 21914241.
Courville AB, Majchrzak-Hong S, Yang S, Turner S, Wilhite B, Ness Shipley K, Horneffer Y, Domenichiello AF, Schwandt M, Cutler RG, Chen KY, Hibbeln JR, Ramsden CE. Dietary linoleic acid lowering alone does not lower arachidonic acid or endocannabinoids among women with overweight and obesity: A randomized, controlled trial. Lipids. 2023 Nov;58(6):271-284. doi: 10.1002/lipd.12382.
Cravotto C, Fabiano-Tixier AS, Claux O, Abert-Vian M, Tabasso S, Cravotto G, Chemat F. Towards Substitution of Hexane as Extraction Solvent of Food Products and Ingredients with No Regrets. Foods. 2022 Oct 28;11(21):3412. doi: 10.3390/foods11213412.
Das UN, Madhavi N. Effect of polyunsaturated fatty acids on drug-sensitive and resistant tumor cells in vitro. Lipids Health Dis. 2011 Sep 14;10:159. doi: 10.1186/1476-511X-10-159.
Dayton S, Pearce ML, Goldman H, Harnish A, Plotkin D, Shickman M, Winfield M, Zager A, Dixon W. Controlled trial of a diet high in unsaturated fat for prevention of atherosclerotic complications. Lancet. 1968 Nov 16;2(7577):1060-2. doi: 10.1016/s0140-6736(68)91531-6.
de Oliveira Otto MC, Wu JH, Baylin A, Vaidya D, Rich SS, Tsai MY, Jacobs DR Jr, Mozaffarian D. Circulating and dietary omega-3 and omega-6 polyunsaturated fatty acids and incidence of CVD in the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2013 Dec 18;2(6):e000506. doi: 10.1161/JAHA.113.000506.
Djuricic I, Calder PC. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients. 2021 Jul 15;13(7):2421. doi: 10.3390/nu13072421.
Fan YY, Ramos KS, Chapkin RS. Dietary gamma-linolenic acid modulates macrophage-vascular smooth muscle cell interactions. Evidence for a macrophage-derived soluble factor that downregulates DNA synthesis in smooth muscle cells. Arterioscler Thromb Vasc Biol. 1995 Sep;15(9):1397-403. doi: 10.1161/01.atv.15.9.1397.
Farvid MS, Ding M, Pan A, Sun Q, Chiuve SE, Steffen LM, Willett WC, Hu FB. Dietary linoleic acid and risk of coronary heart disease: a systematic review and meta-analysis of prospective cohort studies. Circulation. 2014 Oct 28;130(18):1568-78. doi: 10.1161/CIRCULATIONAHA.114.010236.
Ferreiro-Vera C, Priego-Capote F, Mata-Granados JM, Luque de Castro MD. Short-term comparative study of the influence of fried edible oils intake on the metabolism of essential fatty acids in obese individuals. Food Chem. 2013 Jan 15;136(2):576-84. doi: 10.1016/j.foodchem.2012.08.081.
Frantz ID Jr, Dawson EA, Ashman PL, Gatewood LC, Bartsch GE, Kuba K, Brewer ER. Test of effect of lipid lowering by diet on cardiovascular risk. The Minnesota Coronary Survey. Arteriosclerosis. 1989 Jan-Feb;9(1):129-35. doi: 10.1161/01.atv.9.1.129.
Fritsche KL. Linoleic acid, vegetable oils & inflammation. Mo Med. 2014 Jan-Feb;111(1):41-3. PMID: 24645297; PMCID: PMC6179509.
Froyen E, Burns-Whitmore B. The Effects of Linoleic Acid Consumption on Lipid Risk Markers for Cardiovascular Disease in Healthy Individuals: A Review of Human Intervention Trials. Nutrients. 2020 Aug 4;12(8):2329. doi: 10.3390/nu12082329.
Gallagher H, Williams JO, Ferekidis N, Ismail A, Chan YH, Michael DR, Guschina IA, Tyrrell VJ, O’Donnell VB, Harwood JL, Khozin-Goldberg I, Boussiba S, Ramji DP. Dihomo-γ-linolenic acid inhibits several key cellular processes associated with atherosclerosis. Biochim Biophys Acta Mol Basis Dis. 2019 Sep 1;1865(9):2538-2550. doi: 10.1016/j.bbadis.2019.06.011.
Gharby S. Refining Vegetable Oils: Chemical and Physical Refining. ScientificWorldJournal. 2022 Jan 11;2022:6627013. doi: 10.1155/2022/6627013.
Ghoneim DH, Zhu J, Zheng W, Long J, Murff HJ, Ye F, Setiawan VW, Wilkens LR, Khankari NK, Haycock P, Antwi SO, Yang Y, Arslan AA, Beane Freeman LE, Bracci PM, Canzian F, Du M, Gallinger S, Giles GG, Goodman PJ, Kooperberg C, Le Marchand L, Neale RE, Scelo G, Visvanathan K, White E, Albanes D, Amiano P, Andreotti G, Babic A, Bamlet WR, Berndt SI, Brais LK, Brennan P, Bueno-de-Mesquita B, Buring JE, Campbell PT, Rabe KG, Chanock SJ, Duggal P, Fuchs CS, Gaziano JM, Goggins MG, Hackert T, Hassan MM, Helzlsouer KJ, Holly EA, Hoover RN, Katske V, Kurtz RC, Lee IM, Malats N, Milne RL, Murphy N, Oberg AL, Porta M, Rothman N, Sesso HD, Silverman DT, Thompson IM Jr, Wactawski-Wende J, Wang X, Wentzensen N, Yu H, Zeleniuch-Jacquotte A, Yu K, Wolpin BM, Jacobs EJ, Duell EJ, Risch HA, Petersen GM, Amundadottir LT, Kraft P, Klein AP, Stolzenberg-Solomon RZ, Shu XO, Wu L. Mendelian Randomization Analysis of n-6 Polyunsaturated Fatty Acid Levels and Pancreatic Cancer Risk. Cancer Epidemiol Biomarkers Prev. 2020 Dec;29(12):2735-2739. doi: 10.1158/1055-9965.EPI-20-0651.
Grattan BJ Jr. Plant sterols as anticancer nutrients: evidence for their role in breast cancer. Nutrients. 2013 Jan 31;5(2):359-87. doi: 10.3390/nu5020359.
Halvorsen BL, Blomhoff R. Determination of lipid oxidation products in vegetable oils and marine omega-3 supplements. Food Nutr Res. 2011;55. doi: 10.3402/fnr.v55i0.5792.
Harris WS, Mozaffarian D, Rimm E, Kris-Etherton P, Rudel LL, Appel LJ, Engler MM, Engler MB, Sacks F. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation. 2009 Feb 17;119(6):902-7. doi: 10.1161/CIRCULATIONAHA.108.191627.
Hassan STS. Brassicasterol with Dual Anti-Infective Properties against HSV-1 and Mycobacterium tuberculosis, and Cardiovascular Protective Effect: Nonclinical In Vitro and In Silico Assessments. Biomedicines. 2020 May 24;8(5):132. doi: 10.3390/biomedicines8050132.
Hester AG, Murphy RC, Uhlson CJ, Ivester P, Lee TC, Sergeant S, Miller LR, Howard TD, Mathias RA, Chilton FH. Relationship between a common variant in the fatty acid desaturase (FADS) cluster and eicosanoid generation in humans. J Biol Chem. 2014 Aug 8;289(32):22482-9. doi: 10.1074/jbc.M114.579557.
Iggman D, Ärnlöv J, Cederholm T, Risérus U. Association of Adipose Tissue Fatty Acids With Cardiovascular and All-Cause Mortality in Elderly Men. JAMA Cardiol. 2016 Oct 1;1(7):745-753. doi: 10.1001/jamacardio.2016.2259.
Imamura F, Micha R, Wu JH, de Oliveira Otto MC, Otite FO, Abioye AI, Mozaffarian D. Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-analysis of Randomised Controlled Feeding Trials. PLoS Med. 2016 Jul 19;13(7):e1002087. doi: 10.1371/journal.pmed.1002087.
Johnson GH, Fritsche K. Effect of dietary linoleic acid on markers of inflammation in healthy persons: a systematic review of randomized controlled trials. J Acad Nutr Diet. 2012 Jul;112(7):1029-41, 1041.e1-15. doi: 10.1016/j.jand.2012.03.029.
Kernoff PB, Willis AL, Stone KJ, Davies JA, McNicol GP. Antithrombotic potential of dihomo-gamma-linolenic acid in man. Br Med J. 1977 Dec 3;2(6100):1441-4. doi: 10.1136/bmj.2.6100.1441.
Lankinen MA, Fauland A, Shimizu BI, Ågren J, Wheelock CE, Laakso M, Schwab U, Pihlajamäki J. Inflammatory response to dietary linoleic acid depends on FADS1 genotype. Am J Clin Nutr. 2019 Jan 1;109(1):165-175. doi: 10.1093/ajcn/nqy287.
Lankinen MA, Fauland A, Shimizu BI, Ågren J, Wheelock CE, Laakso M, Schwab U, Pihlajamäki J. Inflammatory response to dietary linoleic acid depends on FADS1 genotype. Am J Clin Nutr. 2019 Jan 1;109(1):165-175. doi: 10.1093/ajcn/nqy287.
Leren P. The Oslo diet-heart study. Eleven-year report. Circulation. 1970 Nov;42(5):935-42. doi: 10.1161/01.cir.42.5.935.
Li J, Guasch-Ferré M, Li Y, Hu FB. Dietary intake and biomarkers of linoleic acid and mortality: systematic review and meta-analysis of prospective cohort studies. Am J Clin Nutr. 2020 Jul 1;112(1):150-167. doi: 10.1093/ajcn/nqz349.
Li X, Nian BB, Tan CP, Liu YF, Xu YJ. Deep-frying oil induces cytotoxicity, inflammation and apoptosis on intestinal epithelial cells. J Sci Food Agric. 2022 Jun;102(8):3160-3168. doi: 10.1002/jsfa.11659.
Ligor M, Buszewski B. The comparison of solid phase microextraction-GC and static headspace-GC for determination of solvent residues in vegetable oils. J Sep Sci. 2008 Feb;31(2):364-71. doi: 10.1002/jssc.200700303.
Marklund M, Leander K, Vikström M, Laguzzi F, Gigante B, Sjögren P, Cederholm T, de Faire U, Hellénius ML, Risérus U. Polyunsaturated Fat Intake Estimated by Circulating Biomarkers and Risk of Cardiovascular Disease and All-Cause Mortality in a Population-Based Cohort of 60-Year-Old Men and Women. Circulation. 2015 Aug 18;132(7):586-94. doi: 10.1161/CIRCULATIONAHA.115.015607.
Marklund M, Wu JHY, Imamura F, Del Gobbo LC, Fretts A, de Goede J, Shi P, Tintle N, Wennberg M, Aslibekyan S, Chen TA, de Oliveira Otto MC, Hirakawa Y, Eriksen HH, Kröger J, Laguzzi F, Lankinen M, Murphy RA, Prem K, Samieri C, Virtanen J, Wood AC, Wong K, Yang WS, Zhou X, Baylin A, Boer JMA, Brouwer IA, Campos H, Chaves PHM, Chien KL, de Faire U, Djoussé L, Eiriksdottir G, El-Abbadi N, Forouhi NG, Michael Gaziano J, Geleijnse JM, Gigante B, Giles G, Guallar E, Gudnason V, Harris T, Harris WS, Helmer C, Hellenius ML, Hodge A, Hu FB, Jacques PF, Jansson JH, Kalsbeek A, Khaw KT, Koh WP, Laakso M, Leander K, Lin HJ, Lind L, Luben R, Luo J, McKnight B, Mursu J, Ninomiya T, Overvad K, Psaty BM, Rimm E, Schulze MB, Siscovick D, Skjelbo Nielsen M, Smith AV, Steffen BT, Steffen L, Sun Q, Sundström J, Tsai MY, Tunstall-Pedoe H, Uusitupa MIJ, van Dam RM, Veenstra J, Monique Verschuren WM, Wareham N, Willett W, Woodward M, Yuan JM, Micha R, Lemaitre RN, Mozaffarian D, Risérus U; Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCE). Biomarkers of Dietary Omega-6 Fatty Acids and Incident Cardiovascular Disease and Mortality. Circulation. 2019 May 21;139(21):2422-2436. doi: 10.1161/CIRCULATIONAHA.118.038908.
Mata P, Alonso R, Lopez-Farre A, Ordovas JM, Lahoz C, Garces C, Caramelo C, Codoceo R, Blazquez E, de Oya M. Effect of dietary fat saturation on LDL oxidation and monocyte adhesion to human endothelial cells in vitro. Arterioscler Thromb Vasc Biol. 1996 Nov;16(11):1347-55. doi: 10.1161/01.atv.16.11.1347.
Mazidi M, Shekoohi N, Katsiki N, Banach M. Omega-6 fatty acids and the risk of cardiovascular disease: insights from a systematic review and meta-analysis of randomized controlled trials and a Mendelian randomization study. Arch Med Sci. 2021 Apr 24;18(2):466-479. doi: 10.5114/aoms/136070.
Mirmiran P, Hosseinpour-Niazi S, Naderi Z, Bahadoran Z, Sadeghi M, Azizi F. Association between interaction and ratio of ω-3 and ω-6 polyunsaturated fatty acid and the metabolic syndrome in adults. Nutrition. 2012 Sep;28(9):856-63. doi: 10.1016/j.nut.2011.11.031.
Mo S, Dong L, Hurst WJ, van Breemen RB. Quantitative analysis of phytosterols in edible oils using APCI liquid chromatography-tandem mass spectrometry. Lipids. 2013 Sep;48(9):949-56. doi: 10.1007/s11745-013-3813-3.
Mousavi SM, Jalilpiran Y, Karimi E, Aune D, Larijani B, Mozaffarian D, Willett WC, Esmaillzadeh A. Dietary Intake of Linoleic Acid, Its Concentrations, and the Risk of Type 2 Diabetes: A Systematic Review and Dose-Response Meta-analysis of Prospective Cohort Studies. Diabetes Care. 2021 Sep;44(9):2173-2181. doi: 10.2337/dc21-0438.
Nagai T, Honda Y, Sugano Y, Nishimura K, Nakai M, Honda S, Iwakami N, Okada A, Asaumi Y, Aiba T, Noguchi T, Kusano K, Ogawa H, Yasuda S, Anzai T; NaDEF investigators. Circulating Omega-6, But Not Omega-3 Polyunsaturated Fatty Acids, Are Associated with Clinical Outcomes in Patients with Acute Decompensated Heart Failure. PLoS One. 2016 Nov 8;11(11):e0165841. doi: 10.1371/journal.pone.0165841.
Nilsen DWT, Aarsetoey H, Pönitz V, Brugger-Andersen T, Staines H, Harris WS, Grundt H. The prognostic utility of dihomo-gamma-linolenic acid (DGLA) in patients with acute coronary heart disease. Int J Cardiol. 2017 Dec 15;249:12-17. doi: 10.1016/j.ijcard.2017.09.202.
Nilsen DWT, Myhre PL, Kalstad A, Schmidt EB, Arnesen H, Seljeflot I. Serum Levels of Dihomo-Gamma (γ)-Linolenic Acid (DGLA) Are Inversely Associated with Linoleic Acid and Total Death in Elderly Patients with a Recent Myocardial Infarction. Nutrients. 2021 Sep 30;13(10):3475. doi: 10.3390/nu13103475.
Othman RA, Moghadasian MH. Beyond cholesterol-lowering effects of plant sterols: clinical and experimental evidence of anti-inflammatory properties. Nutr Rev. 2011 Jul;69(7):371-82. doi: 10.1111/j.1753-4887.2011.00399.x.
Özcan MM, Juhaimi FA, Uslu N, Ghafoor K, Ahmed IAM, Babiker EE. The Effect of Olive Varieties on Fatty Acid Composition and Tocopherol Contents of Cold Pressed Virgin Olive Oils. J Oleo Sci. 2019;68(4):307-310. doi: 10.5650/jos.ess18251.
Palazhy S, Kamath P, Vasudevan DM. Dietary Fats and Oxidative Stress: A Cross-Sectional Study Among Coronary Artery Disease Subjects Consuming Coconut Oil/Sunflower Oil. Indian J Clin Biochem. 2018 Jan;33(1):69-74. doi: 10.1007/s12291-017-0639-4. Epub 2017 Feb 1.
Park S, Lee S, Kim Y, Lee Y, Kang M, Kim K, Kim Y, Han S, Lee H, Lee J, Joo K, Lim C, Kim Y, Kim D. Causal Effects of Serum Levels of n-3 or n-6 Polyunsaturated Fatty Acids on Coronary Artery Disease: Mendelian Randomization Study. Nutrients. 2021 Apr 28;13(5):1490. doi: 10.3390/nu13051490.
Pischon T, Hankinson SE, Hotamisligil GS, Rifai N, Willett WC, Rimm EB. Habitual dietary intake of n-3 and n-6 fatty acids in relation to inflammatory markers among US men and women. Circulation. 2003 Jul 15;108(2):155-60. doi: 10.1161/01.CIR.0000079224.46084.C2.
Poudel-Tandukar K, Nanri A, Matsushita Y, Sasaki S, Ohta M, Sato M, Mizoue T. Dietary intakes of alpha-linolenic and linoleic acids are inversely associated with serum C-reactive protein levels among Japanese men. Nutr Res. 2009 Jun;29(6):363-70. doi: 10.1016/j.nutres.2009.05.012.
Princen HM, van Duyvenvoorde W, Buytenhek R, van der Laarse A, van Poppel G, Gevers Leuven JA, van Hinsbergh VW. Supplementation with low doses of vitamin E protects LDL from lipid peroxidation in men and women. Arterioscler Thromb Vasc Biol. 1995 Mar;15(3):325-33. doi: 10.1161/01.atv.15.3.325.
Rabehl M, Wei Z, Leineweber CG, Enssle J, Rothe M, Jung A, Schmöcker C, Elbelt U, Weylandt KH, Pietzner A. Effect of FADS1 SNPs rs174546, rs174547 and rs174550 on blood fatty acid profiles and plasma free oxylipins. Front Nutr. 2024 Jul 3;11:1356986. doi: 10.3389/fnut.2024.1356986.
Raederstorff D, Wyss A, Calder PC, Weber P, Eggersdorfer M. Vitamin E function and requirements in relation to PUFA. Br J Nutr. 2015 Oct 28;114(8):1113-22. doi: 10.1017/S000711451500272X.
Ramsden CE, Zamora D, Leelarthaepin B, Majchrzak-Hong SF, Faurot KR, Suchindran CM, Ringel A, Davis JM, Hibbeln JR. Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis. BMJ. 2013 Feb 4;346:e8707. doi: 10.1136/bmj.e8707.
Reaven P, Parthasarathy S, Grasse BJ, Miller E, Almazan F, Mattson FH, Khoo JC, Steinberg D, Witztum JL. Feasibility of using an oleate-rich diet to reduce the susceptibility of low-density lipoprotein to oxidative modification in humans. Am J Clin Nutr. 1991 Oct;54(4):701-6. doi: 10.1093/ajcn/54.4.701.
Reaven P, Parthasarathy S, Grasse BJ, Miller E, Steinberg D, Witztum JL. Effects of oleate-rich and linoleate-rich diets on the susceptibility of low density lipoprotein to oxidative modification in mildly hypercholesterolemic subjects. J Clin Invest. 1993 Feb;91(2):668-76. doi: 10.1172/JCI116247.
Reaven PD, Grasse BJ, Tribble DL. Effects of linoleate-enriched and oleate-enriched diets in combination with alpha-tocopherol on the susceptibility of LDL and LDL subfractions to oxidative modification in humans. Arterioscler Thromb. 1994 Apr;14(4):557-66. doi: 10.1161/01.atv.14.4.557.
Rett BS, Whelan J. Increasing dietary linoleic acid does not increase tissue arachidonic acid content in adults consuming Western-type diets: a systematic review. Nutr Metab (Lond). 2011 Jun 10;8:36. doi: 10.1186/1743-7075-8-36.
Samsuri AH, Ang MY, Ng SY. Optimization of Residual Hexane in Edible Oils Analysis Using Static Headspace Gas Chromatography. Int J Anal Chem. 2021 Oct 29;2021:1941336. doi: 10.1155/2021/1941336.
Seah JY, Gay GM, Su J, Tai ES, Yuan JM, Koh WP, Ong CN, van Dam RM. Consumption of Red Meat, but Not Cooking Oils High in Polyunsaturated Fat, Is Associated with Higher Arachidonic Acid Status in Singapore Chinese Adults. Nutrients. 2017 Jan 31;9(2):101. doi: 10.3390/nu9020101.
Sergeant S, Hallmark B, Mathias RA, Mustin TL, Ivester P, Bohannon ML, Ruczinski I, Johnstone L, Seeds MC, Chilton FH. Prospective clinical trial examining the impact of genetic variation in FADS1 on the metabolism of linoleic acid- and ɣ-linolenic acid-containing botanical oils. Am J Clin Nutr. 2020 May 1;111(5):1068-1078. doi: 10.1093/ajcn/nqaa023.
Sergeant S, Rahbar E, Chilton FH. Gamma-linolenic acid, Dihommo-gamma linolenic, Eicosanoids and Inflammatory Processes. Eur J Pharmacol. 2016 Aug 15;785:77-86. doi: 10.1016/j.ejphar.2016.04.020.
Shen J, Liu Y, Wang X, Bai J, Lin L, Luo F, Zhong H. A Comprehensive Review of Health-Benefiting Components in Rapeseed Oil. Nutrients. 2023 Feb 16;15(4):999. doi: 10.3390/nu15040999.
Tsimikas S, Philis-Tsimikas A, Alexopoulos S, Sigari F, Lee C, Reaven PD. LDL isolated from Greek subjects on a typical diet or from American subjects on an oleate-supplemented diet induces less monocyte chemotaxis and adhesion when exposed to oxidative stress. Arterioscler Thromb Vasc Biol. 1999 Jan;19(1):122-30. doi: 10.1161/01.atv.19.1.122.
Van den Oever SP, Maruta CK, Schreiner M, Mayer HK. “Exotic” seeds from Southern Africa as potential Novel Foods? – Chemical composition of manketti nuts (Schinziophyton rautanenii) and ushivi beans (Guibourtia coleosperma). Food Res Int. 2024 May;184:114200. doi: 10.1016/j.foodres.2024.114200.
Venø SK, Bork CS, Jakobsen MU, Lundbye-Christensen S, Bach FW, Overvad K, Schmidt EB. Linoleic Acid in Adipose Tissue and Development of Ischemic Stroke: A Danish Case-Cohort Study. J Am Heart Assoc. 2018 Jun 26;7(13):e009820. doi: 10.1161/JAHA.118.009820.
Virtanen JK, Wu JHY, Voutilainen S, Mursu J, Tuomainen TP. Serum n-6 polyunsaturated fatty acids and risk of death: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr. 2018 Mar 1;107(3):427-435. doi: 10.1093/ajcn/nqx063.
Wang X, Lin H, Gu Y. Multiple roles of dihomo-γ-linolenic acid against proliferation diseases. Lipids Health Dis. 2012 Feb 14;11:25. doi: 10.1186/1476-511X-11-25.
Wu JH, Lemaitre RN, King IB, Song X, Psaty BM, Siscovick DS, Mozaffarian D. Response to Letters Regarding Article, “Circulating Omega-6 Polyunsaturated Fatty Acids and Total and Cause-Specific Mortality: The Cardiovascular Health Study”. Circulation. 2015 Jul 21;132(3):e25-6. doi: 10.1161/CIRCULATIONAHA.115.014853.
Wu JH, Lemaitre RN, King IB, Song X, Psaty BM, Siscovick DS, Mozaffarian D. Circulating omega-6 polyunsaturated fatty acids and total and cause-specific mortality: the Cardiovascular Health Study. Circulation. 2014 Oct 7;130(15):1245-53. doi: 10.1161/CIRCULATIONAHA.114.011590.
Wu JHY, Marklund M, Imamura F, Tintle N, Ardisson Korat AV, de Goede J, Zhou X, Yang WS, de Oliveira Otto MC, Kröger J, Qureshi W, Virtanen JK, Bassett JK, Frazier-Wood AC, Lankinen M, Murphy RA, Rajaobelina K, Del Gobbo LC, Forouhi NG, Luben R, Khaw KT, Wareham N, Kalsbeek A, Veenstra J, Luo J, Hu FB, Lin HJ, Siscovick DS, Boeing H, Chen TA, Steffen B, Steffen LM, Hodge A, Eriksdottir G, Smith AV, Gudnason V, Harris TB, Brouwer IA, Berr C, Helmer C, Samieri C, Laakso M, Tsai MY, Giles GG, Nurmi T, Wagenknecht L, Schulze MB, Lemaitre RN, Chien KL, Soedamah-Muthu SS, Geleijnse JM, Sun Q, Harris WS, Lind L, Ärnlöv J, Riserus U, Micha R, Mozaffarian D; Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCE). Omega-6 fatty acid biomarkers and incident type 2 diabetes: pooled analysis of individual-level data for 39 740 adults from 20 prospective cohort studies. Lancet Diabetes Endocrinol. 2017 Dec;5(12):965-974. doi: 10.1016/S2213-8587(17)30307-8.
Yahay M, Heidari Z, Allameh Z, Amani R. The effects of canola and olive oils consumption compared to sunflower oil, on lipid profile and hepatic steatosis in women with polycystic ovarian syndrome: a randomized controlled trial. Lipids Health Dis. 2021 Jan 29;20(1):7. doi: 10.1186/s12944-021-01433-9.
Yang R, Xue L, Zhang L, Wang X, Qi X, Jiang J, Yu L, Wang X, Zhang W, Zhang Q, Li P. Phytosterol Contents of Edible Oils and Their Contributions to Estimated Phytosterol Intake in the Chinese Diet. Foods. 2019 Aug 9;8(8):334. doi: 10.3390/foods8080334.
Yang T, Yi J, He Y, Zhang J, Li X, Ke S, Xia L, Liu L. Associations of Dietary Fats with All-Cause Mortality and Cardiovascular Disease Mortality among Patients with Cardiometabolic Disease. Nutrients. 2022 Aug 31;14(17):3608. doi: 10.3390/nu14173608.
Ye D, Huang H, Wu DJH, Zhang W, Zhou F, Qian Y, Zheng J, Mao Y. Association Between Circulating Linoleic Acid and Risk of Ischemic Stroke. Front Genet. 2021 Jan 8;11:582623. doi: 10.3389/fgene.2020.582623.
Zeng J, Wu J, Pang S, Wang F, Yu X, Zhang S, Zeng J, Yan J, Lian J. Brassicasterol inhibits hepatitis B virus-associated hepatocellular carcinoma development via suppression of AKT signaling pathway. Infect Agent Cancer. 2023 Apr 20;18(1):22. doi: 10.1186/s13027-023-00502-1.
Zhang W, Zhou F, Huang H, Mao Y, Ye D. Biomarker of dietary linoleic acid and risk for stroke: A systematic review and meta-analysis. Nutrition. 2020 Nov-Dec;79-80:110953. doi: 10.1016/j.nut.2020.110953.
Zhang Y, Guo X, Gao J, Wei C, Zhao S, Liu Z, Sun H, Wang J, Liu L, Li Y, Han T, Sun C. The associations of circulating common and uncommon polyunsaturated fatty acids and modification effects on dietary quality with all-cause and disease-specific mortality in NHANES 2003-2004 and 2011-2012. Ann Med. 2021 Dec;53(1):1744-1757. doi: 10.1080/07853890.2021.1937693.
Zhang Y, Sun Y, Yu Q, Song S, Brenna JT, Shen Y, Ye K. Higher ratio of plasma omega-6/omega-3 fatty acids is associated with greater risk of all-cause, cancer, and cardiovascular mortality: A population-based cohort study in UK Biobank. Elife. 2024 Apr 5;12:RP90132. doi: 10.7554/eLife.90132.
Zhou Y, Wang T, Zhai S, Li W, Meng Q. Linoleic acid and breast cancer risk: a meta-analysis. Public Health Nutr. 2016 Jun;19(8):1457-63. doi: 10.1017/S136898001500289X.



