Key Takeaways (expand)
- Alpha-linolenic acid (ALA) is the most abundant omega-3 fatty acid in most people’s diets, and the only omega-3 that’s truly essential.
- ALA serves as a precursor for two other important omega-3 fats, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)—although the conversion rate is fairly low.
- Because ALA competes with the omega-6 fat linoleic acid for some of the same conversion enzymes, a high linoleic acid intake can reduce the amount of EPA and DHA that gets created from ALA.
- Like other omega-3 fats, ALA helps produce signaling molecules called eicosanoids that regulate inflammation, blood pressure, and pain perception.
- ALA also gets incorporated into cell membranes, where it alters important structural properties like fluidity, permeability, and flexibility.
- ALA can be used to create ketone bodies, burned for energy, or stored in adipose tissue.
- Consuming enough ALA can boost cardiovascular health—including improving risk factors (like triglycerides, LDL cholesterol, and high blood pressure) and actual cardiovascular outcomes (including heart attacks, heart arrhythmia, and coronary heart disease mortality).
- ALA consumption is also associated with a reduced risk of death from all causes.
- Although more controlled human trials are needed, observational studies, animal experiments, and in vitro research suggest ALA could be cancer-protective.
- Higher ALA intake is associated with a lower risk of a number of other health conditions, including multiple sclerosis relapse, depression, pneumonia, and stomach ulcers.
- Chronically low ALA intake (especially when omega-6 intake is high) is associated with a higher risk of cardiovascular disease, obesity, and inflammatory skin conditions.
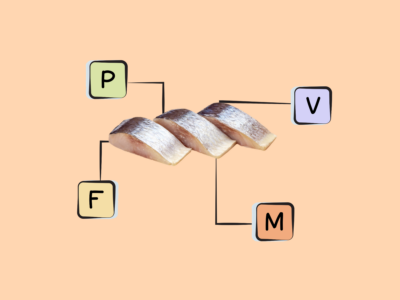
- Great sources of ALA include many seeds, nuts, and their oils, such as flaxseed, hempseed, chia seeds, walnuts, pistachios, pumpkin seeds, and oils made from them; other vegetable oils (like soybean oil and canola/rapeseed oil) also contain ALA.
Alpha-linolenic acid, more often referred to as ALA, is the most abundant omega-3 essential fatty acid in most Western diets (not to be confused with two other similarly named fats, linoleic acid and linolenic acid!). It was first discovered in 1887 by an Austrian chemist named Karl Hazura, but its chemical structure wasn’t fully clarified until 1909. The name alpha-linolenic acid comes from the Latin words linum, meaning “flax,” and oleic, meaning “pertaining to oil”—referring to its initial isolation from flaxseed oil.
Along with providing energy to the body, ALA is needed for normal human growth and development, and also plays a role in antioxidant defense and cardiovascular health. It serves as a precursor for two other omega-3 fats, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA).
The best food sources of ALA are flaxseed, hempseed, chia seeds, and their oils; it’s also found in walnuts, pistachios, pumpkin seeds, and other vegetable oils (like soybean oil and canola/rapeseed oil).
Want to know the top 25 foods for this awesome nutrient?

The Top 25 Foods for Every Nutrient
The Top 25 Foods for Every Nutrient e-book is a well-organized, easy-to-use, grocery store-friendly guide to help you choose foods that fit your needs of 43 important nutrients while creating a balanced nutrient-dense diet.
Get two “Top 25” food lists for each nutrient, plus you’ll find RDA charts for everyone, informative visuals, fun facts, serving sizes and the 58 foods that are Nutrient Super Stars!
Buy now for instant digital access.
The Biological Roles of ALA
All omega-3 fats are characterized by a shared feature in their chemical structure: a double bond located three atoms away from their terminal methyl group. They also contain multiple other double bonds, making them “polyunsaturated” fats (in contrast to monounsaturated fats, which have only one double bond, or saturated fats, which have none). In general, omega-3 fats are used for chemical reactions within cells that produce signaling molecules involved in inflammation, blood pressure regulation, and pain perception. They’re components of the cell membrane, where they alter important structural properties like membrane permeability, fluidity, and flexibility—consequently impacting the activity of membrane receptor systems and influencing gene expression and signaling pathways. The omega-3s we consume from food alter the proportion of omega-3 fats that end up in red blood cells, cardiac tissue, immune cells, atherosclerotic plaque, and more, in turn affecting the characteristics of these cells and the function of their receptors!
Omega-3 fats compete with omega-6 fats for the same conversion enzymes, as well as for positions in cell membranes—which is why we often hear about the importance of a balanced ratio between these two families. Too much dietary omega-6 relative to omega-3 can interfere with omega-3 metabolism, and is associated with a number of chronic health conditions such as obesity, cardiovascular disease, Alzheimer’s disease, non-alcoholic fatty liver disease, inflammatory bowel disease, and rheumatoid arthritis. Historically, the human diet is estimated to have featured about a 1:1 ratio of omega-6 to omega-3 fats, whereas the modern Western diet has a ratio of 15 to 20:1 or higher (largely due to the increased consumption of omega-6 rich grains and vegetable oils, and under-consumption of omega-3 rich seafood)!
Although the most common omega-3 fats we hear about are ALA, EPA, and DHA, there are actually 11 different omega fats in total! But, these “big three” are considered most important for human health, and have been the subject of far more research than the lesser-known omega-3 fats.
Importantly, ALA is the only omega-3 fat that’s considered truly essential, because the body can’t synthesize it any way; EPA and DHA can actually be made from ALA! More specifically, when we ingest ALA, some of it undergoes a series of desaturation and elongation reactions (the addition of double bonds and carbon atoms, respectively) that results in the creation of EPA, which can then be further converted to DHA. But, this process is very inefficient, and can vary based on a person’s gender, genetics, and overall diet.
For example, women have a higher conversion capacity than men: in studies of healthy young male participants, only about 8% of dietary ALA was converted to EPA and 0-4% was converted to DHA; in healthy young women, about 21% of ALA was converted to EPA while 9% was converted to DHA. These differences appear due to the effects of estrogen on a specific nuclear receptor protein (called peroxisome proliferator-activated receptor alpha, or PPAR-α), which then alters the expression of the enzymes that participate in EPA and DHA synthesis. It’s likely women developed a greater capacity for ALA conversion due to the increased need for EPA and DHA during pregnancy and lactation.
Genetics can also influence how much ALA a person can convert. For example, two common haplotypes (clusters of gene variants) involving the fatty acid desaturase (FADS) genes significantly influence how efficiently EPA and DHA are synthesized, and are also major determinants of circulating levels of omega-3s within the body. In fact, FADS polymorphisms may explain up to 30% of the individual variation in blood concentrations of omega-3 fats! Research has shown that ethnic background is particularly associated with these variations: for example, people of Indigenous American descent are much more likely to carry FADS variants that limit the conversion of omega-3 fats, in turn making them more susceptible to deficiency or insufficiency.
Other dietary components can likewise impact how much ALA the body can convert. ALA competes with an omega-6 fat, linoleic acid, for the same elongase and desaturase enzymes; as a result, high linoleic acid intake can drop the conversion of ALA to EPA and DHA by as much as 40%. Trans fatty acids, saturated fats, and dietary cholesterol can also interfere with the desaturation and elongation processes used to convert ALA to EPA and DHA. Interestingly, studies have suggested that exposure to cigarette smoke can negatively impact ALA conversion, too!
After being consumed, dietary ALA has several different metabolic fates. Along with a small amount being converted to EPA and DHA, ALA can be directly incorporated into the phospholipids of cell membranes, used to produce ketone bodies, or stored in adipose tissue. And, a significant amount of ingested ALA (24 to 33% of ALA in men and 19 to 22% in women) undergoes beta-oxidation to produce energy available for immediate use, which then fuels essential body processes like breathing, digestion, and muscle function.
Due to their molecular structure, all omega-3 fats are relatively prone to oxidation: their multiple double bonds can attract electrons from nearby carbons, resulting in oxidation. This is important to keep in mind, because some studies of dietary omega-3 intake can be confounded by the way omega-3-rich foods or supplements are prepared or stored.
Everything You Need to Jump into Nutrivore TODAY!

Nutrivore Quickstart Guide
The Nutrivore Quickstart Guide e-book explains why and how to eat a Nutrivore diet, introduces the Nutrivore Score, gives a comprehensive tour of the full range of essential and important nutrients!
Plus, you’ll find the Top 100 Nutrivore Score Foods, analysis of food groups, practical tips to increase the nutrient density of your diet, and look-up tables for the Nutrivore Score of over 700 foods.
Buy now for instant digital access.
ALA in Health and Disease
Because it can be converted to EPA and DHA, some of the health effects associated with ALA consumption are due to its contribution to the body’s supply of these other two omega-3 fats. However, ALA has also been independently linked to its own health benefits, particularly when it comes to cardiovascular disease and cancer!
ALA and Cardiovascular Disease
A number of studies point to a protective effect of ALA on the heart. Clinical trials have shown that ALA supplementation can improve cardiovascular risk factors like triglyceride levels, total cholesterol, low-density lipoprotein or LDL cholesterol (especially in people with diabetes or metabolic syndrome), and the triglyceride to HDL ratio. And, observational research has linked higher dietary ALA intake with lower incidence of high blood pressure, less plaque in the arteries, and healthier heart rhythm (as measured by QT and JT intervals). All of these suggest a benefit of ALA on the cardiovascular system.
Likewise, ALA is generally associated with better cardiovascular disease outcomes. A comprehensive review of the highest-quality randomized controlled trials of omega-3 intake (all lasting at least 12 months, and with a combined total of over 112,000 participants!) found that ALA consumption slightly reduced the risk of cardiovascular disease events, coronary heart disease mortality, and heart arrhythmia. Likewise, a very large systematic review and meta-analysis, encompassing nearly 1.2 million participants across different studies, found that high (versus low) dietary ALA intake was associated with a 10% reduced risk of death from all causes, an 11% reduced risk of death from coronary heart disease, and an 8% reduced risk of death from cardiovascular disease. Analyzed another way, every 1 g daily increase in ALA intake (about the same as a half-ounce of walnut, or one tablespoon of canola oil) was associated with a 5% drop in both cardiovascular disease mortality and all-cause mortality. Higher blood levels of ALA were likewise associated with reduced all-cause and coronary heart disease mortality in this analysis. Another meta-analysis of prospective cohort studies (which follow certain groups of people over time) echoed these findings, showing that higher ALA intake was associated with a 10% lower risk of cardiovascular disease mortality and a 7% reduced risk of death from all causes.
But, some of the research has been conflicting: another meta-analysis of cohort studies found that while higher versus lower ALA intake generally seemed heart-protective, there was also a J-shaped curve where intakes of over 1.4 g of ALA daily started correlating with an excess of heart disease incidence and death—suggesting too much ALA could also be a problem. More research here may be needed to explore this possibility.
Because the biggest contributors of ALA to most people’s diets (vegetable oils) are also high in other fatty acids, particularly a potentially pro-inflammatory omega-6 fat called linoleic acid, it’s possible that observational studies are affected by confounding from other nutrient intakes. Likewise, studies differ in how they assess ALA intake, with some using dietary surveys and some measuring blood or tissue levels as a proxy for dietary intake. This is important, because not all measures are equally accurate (for example, tissue content of ALA can be influenced by a person’s metabolism, by differences in laboratory assays, and even by variations that occur during sample storage!) Indeed, some studies have found no association between ALA and cardiovascular disease outcomes when using tissue samples, but an inverse link when using blood samples or dietary surveys.
We do know that mechanistically, ALA would be expected to benefit cardiovascular health due to its anticoagulant properties, triglyceride and blood-pressure-lowering effects, ability to regulate the production of eicosanoids (inflammation-mediating molecules) from omega-6 fats, effects on blood vessel function, and influence on ion influx within cardiac cells—among other potential roles. Combined with promising results from the highest-quality clinical trials, it would be reasonable to say that on the whole, ALA looks good for the heart!
ALA and Cancer
Additional research suggests that ALA may be cancer-protective. In observational studies of people with cancer versus healthy controls, higher blood levels of ALA have been associated with a 57% reduced incidence of colon cancer and a 59% reduced incidence of rectal cancer. Likewise, women with higher ALA content in their breast tissue appear less likely to develop breast cancer over time, and for those who already have the disease, have a dramatically lower risk (80%) of the cancer spreading to other parts of their body. And in postmenopausal women with breast cancer, the addition of 25 g daily of flaxseed (which has over half its fatty acids in the form of ALA) was able to significantly reduce cell proliferation and increase cell death (apoptosis) in their tumors. Of course, given that flaxseed oil contains other potentially anti-cancer components such as lignans, it’s hard to ascribe these results to the ALA alone.
A 2021 study of organ transplant recipients, a population known to have significantly (100-fold!) higher risk of developing skin cancer, found that ALA intake was protective against basal cell carcinoma. Specifically, patients with high ALA intake had a 60% lower risk of this form of skin cancer.
Although caution is needed when translating the results to humans, cell studies have also shown a protective effect of ALA on cancer growth. For example, in various experiments, pure ALA was able to inhibit the proliferation of breast cancer cells, esophageal cancer cells, bladder cancer cells, colon cancer cells, and prostate cancer cells, while also reducing the adhesion and invasion of some cells. This suggests that ALA itself, rather than just the EPA and DHA it can generate, has anti-cancer activity—at least in vitro!
As with research on cardiovascular disease, though, the findings here are sometimes mixed. An extremely large meta-analysis of ALA and health outcomes, encompassing 1.2 million participants, found that high (versus low) dietary ALA intake was associated with a 6% increased risk of cancer mortality. However, a dose-response analysis of the data showed no increased risk when ALA intake was between 0.27 g to 3 g daily, and there was no association at all between cancer mortality and tissue concentrations of ALA (a potentially more reliable measurement than dietary recall surveys). Multiple studies have linked greater concentrations of ALA in prostate tissue to more aggressive prostate cancer, as well as to higher levels of prostate-specific antigen (a protein that’s often elevated in people with prostate cancer). Similarly, a meta-analysis of 47 randomized trials concluded that higher ALA intakes were associated with a slightly greater risk of getting diagnosed with prostate cancer. But, the authors of this study noted that the evidence pointing to this trend was potentially low quality and at high risk of publication bias, making its validity uncertain.
It’s also worth noting that any pro-cancer effect of ALA could be due to the existence of trans forms of ALA in some foods (indeed, when studies adjust for this confounder, links with cancer tend to become non-significant), or due to ALA being ingested in an oxidized form (such as from being reused multiple times as a fry oil). In the case of prostate cancer, some evidence suggests the link could be greatly dependent on genetic variations in ALA metabolism; for example, polymorphisms affecting an important fatty acid desaturation enzyme (delta-6 desaturase) have been shown to modify the relationship between prostate tissue ALA concentrations, prostate-specific antigen levels, and tumor proliferation rate. Studies of whole-food ALA sources like walnuts and flaxseed typically show a protective effect against cancer, suggesting that the dietary source could modify disease associations too. And, ALA has several known mechanisms that could feasibly give it an anti-cancer role—such as competing with linoleic acid for desaturation enzymes (in turn and reducing the amount of omega-6 fats available for creating inflammatory eicosanoids), interfering with cell growth by generating peroxidation products, downregulating certain tyrosine kinase receptors (which control a number of cancer-related functions), and down-regulating growth factor-mediated signaling pathways. So, while it’s possible that ALA increases the risk of cancer or specific types of cancer, the evidence suggesting so is weak at best.
ALA and Other Health Conditions
Although ALA has been less thoroughly studied for other conditions, research has also linked higher ALA intake with a reduced risk of pneumonia, stroke, multiple sclerosis-related depression and fatigue, multiple sclerosis relapse, and clinical depression. This fatty acid can also inhibit the growth of the H. pylori bacteria responsible for stomach ulcers. When it comes to stroke and neurological health, a protective effect may be due to ALA’s ability to increase levels of brain-derived neurotrophic factor (BDNF)—a protein with neuroprotective properties, and which various studies have shown could help reduce depression and improve health outcomes following a stroke. In some experiments, ALA has been shown to exert an even more pronounced effect than EPA and DHA on protecting neurons from glutamate-mediated excitotoxic death (a cell death mechanism caused by excessive release of glutamate from neurons). Likewise, ALA may enhance neuronal tolerance to stressful environments like ischemia (or inadequate blood supply) through its effects on a transcription factor called nuclear factor kappaB.
Lastly, human and animal studies of high-ALA foods like flaxseed oil and perilla seed oil have shown a potentially protective effect against asthma, skin diseases like eczema and psoriasis, dry eye, diabetes, PCOS-related elevations in insulin levels, inflammatory bowel disease, kidney function, allergies, alcohol-induced stomach ulcers, and rheumatoid arthritis. However, since these foods also contain other fatty acids and health-promoting polyphenols, it’s unclear how much of these effects were due to ALA alone, other food components, or a synergistic effect of both! (It’s also worth noting that mice and rats much more readily convert ALA to EPA and DHA than humans do, so the results of rodent studies of ALA-rich foods may not accurately reflect what happens for humans!)
Didn’t know ALA was this interesting? Maybe your friends will enjoy this too!
Nutrivore Is a Game-Changer—This FREE Guide Shows You Why
Sign up for the free Nutrivore Newsletter, your weekly, science-backed guide to improving health through nutrient-rich foods — without dieting harder —and get the Beginner’s Guide to Nutrivore delivered straight to your inbox!

Health Effects of ALA Deficiency
A low intake of omega-3 fats in general, especially when omega-6 intake is simultaneously high, is associated with a greater risk of a number of health conditions developing over time (including cardiovascular disease, inflammatory skin conditions, and obesity). However, true omega-3 deficiency (and essential fatty acid deficiency in general) is very rare: even when intake or absorption is low—such as from being on a fat-restricted diet, or having a fat malabsorption disorder—the body is able to release essential fatty acids from fat tissue into circulation, preventing acute deficiency from occurring.
Documented cases of omega-3 deficiency are mostly limited to incidents from the 1970s and 1980s when people received parenteral nutrition (nutrients delivered intravenously) formulated without any polyunsaturated fatty acids, combined with glucose solutions that suppressed the release of essential fatty acids from fat tissue. In these cases, symptoms of classical essential fatty acid deficiency occurred in about 7 to 10 days, and included dry, scaly skin rashes and hair loss. Due to the role of omega-3s in maintaining proper nervous system functioning, chronic deficiency can also lead to numbness, leg pain, difficulty walking, poor growth in children, and blurred vision. But again, these effects are rare, and the biggest problem with omega-3 insufficiency is a higher long-term risk of chronic health issues, rather than any acute effects of deficiency.
Because of its conversion to EPA and DHA, the specific effects of ALA deficiency have been hard to study, and it may not produce deficiency at all if EPA and DHA are ingested directly from other dietary sources like seafood. Issues stemming from ALA deficiency are more often related to deficiencies of its main end product, DHA.
How Much ALA Do We Need?
For preventing deficiency, 0.2 to 0.3% of total calories consumed daily should be from ALA. The adequate intake for ALA is 1.6 g daily for adult men and 1.1 g daily for adult women (1.4 g daily while pregnant, 1.3 g daily whole breast-feeding). However, these intakes should be viewed in the context of high individual variability of ALA conversion, as well as how much omega-6 is also in the diet. In general, consuming a diet that includes ALA sources like walnuts or flaxseed, and/or fatty fish (especially wild caught), should be enough to meet most people’s omega-3 needs.
| 0 – 6 months | |||||
| 6 months to < 12 months | |||||
| 1 yr – 3 yrs | |||||
| 4 yrs – 8 yrs | |||||
| 9 yrs – 13 yrs | |||||
| 14 yrs – 18 yrs | |||||
| 19 yrs – 50 yrs | |||||
| 51+ yrs | |||||
| Pregnant (14 – 18 yrs) | |||||
| Pregnant (19 – 30 yrs) | |||||
| Pregnant (31 – 50 yrs) | |||||
| Lactating (14 – 18 yrs) | |||||
| Lactating (19 – 30 yrs) | |||||
| Lactating (31 – 50 yrs) |
Nutrient Daily Values
Nutrition requirements and recommended nutrient intake for infants, children, adolescents, adults, mature adults, and pregnant and lactating individuals.
Good Food Sources of ALA
The following foods are sources of ALA, containing at least 0.16 grams per serving.
Citations
Expand to see all scientific references for this article.
Abdelhamid AS, Brown TJ, Brainard JS, Biswas P, Thorpe GC, Moore HJ, Deane KH, Summerbell CD, Worthington HV, Song F, Hooper L. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2020 Feb 29;3(3):CD003177. doi: 10.1002/14651858.CD003177.pub5.
Ameur A, Enroth S, Johansson A, Zaboli G, Igl W, Johansson AC, Rivas MA, Daly MJ, Schmitz G, Hicks AA, Meitinger T, Feuk L, van Duijn C, Oostra B, Pramstaller PP, Rudan I, Wright AF, Wilson JF, Campbell H, Gyllensten U. Genetic adaptation of fatty-acid metabolism: a human-specific haplotype increasing the biosynthesis of long-chain omega-3 and omega-6 fatty acids. Am J Hum Genet. 2012 May 4;90(5):809-20. doi: 10.1016/j.ajhg.2012.03.014.
Arm JP, Boyce JA, Wang L, Chhay H, Zahid M, Patil V, Govindarajulu U, Ivester P, Weaver KL, Sergeant S, Israel E, Chilton FH. Impact of botanical oils on polyunsaturated fatty acid metabolism and leukotriene generation in mild asthmatics. Lipids Health Dis. 2013 Oct 2;12:141. doi: 10.1186/1476-511X-12-141.
Azrad M, Zhang K, Vollmer RT, Madden J, Polascik TJ, Snyder DC, Ruffin MT, Moul JW, Brenner D, Hardy RW, Demark-Wahnefried W. Prostatic alpha-linolenic acid (ALA) is positively associated with aggressive prostate cancer: a relationship which may depend on genetic variation in ALA metabolism. PLoS One. 2012;7(12):e53104. doi: 10.1371/journal.pone.0053104.
Blondeau N, Lipsky RH, Bourourou M, Duncan MW, Gorelick PB, Marini AM. Alpha-linolenic acid: an omega-3 fatty acid with neuroprotective properties-ready for use in the stroke clinic? Biomed Res Int. 2015;2015:519830. doi: 10.1155/2015/519830.
Blondeau N, Widmann C, Lazdunski M, Heurteaux C. Polyunsaturated fatty acids induce ischemic and epileptic tolerance. Neuroscience. 2002;109(2):231-41. doi: 10.1016/s0306-4522(01)00473-0.
Bougnoux P, Koscielny S, Chajès V, Descamps P, Couet C, Calais G. alpha-Linolenic acid content of adipose breast tissue: a host determinant of the risk of early metastasis in breast cancer. Br J Cancer. 1994 Aug;70(2):330-4. doi: 10.1038/bjc.1994.302.
Brouwer IA, Geleijnse JM, Klaasen VM, Smit LA, Giltay EJ, de Goede J, Heijboer AC, Kromhout D, Katan MB. Effect of alpha linolenic acid supplementation on serum prostate specific antigen (PSA): results from the alpha omega trial. PLoS One. 2013 Dec 11;8(12):e81519. doi: 10.1371/journal.pone.0081519.
Burdge G. Alpha-linolenic acid metabolism in men and women: nutritional and biological implications. Curr Opin Clin Nutr Metab Care. 2004 Mar;7(2):137-44. doi: 10.1097/00075197-200403000-00006.
Burdge GC, Jones AE, Wootton SA. Eicosapentaenoic and docosapentaenoic acids are the principal products of alpha-linolenic acid metabolism in young men*. Br J Nutr. 2002 Oct;88(4):355-63. doi: 10.1079/BJN2002662.
Burdge GC, Wootton SA. Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br J Nutr. 2002 Oct;88(4):411-20. doi: 10.1079/BJN2002689.
Burdge GC. Metabolism of alpha-linolenic acid in humans. Prostaglandins Leukot Essent Fatty Acids. 2006 Sep;75(3):161-8. doi: 10.1016/j.plefa.2006.05.013.
Burns T, Maciejewski SR, Hamilton WR, Zheng M, Mooss AN, Hilleman DE. Effect of omega-3 fatty acid supplementation on the arachidonic acid:eicosapentaenoic acid ratio. Pharmacotherapy. 2007 May;27(5):633-8. doi: 10.1592/phco.27.5.633.
Butler LM, Yuan JM, Huang JY, Su J, Wang R, Koh WP, Ong CN. Plasma fatty acids and risk of colon and rectal cancers in the Singapore Chinese Health Study. NPJ Precis Oncol. 2017 Nov 23;1(1):38. doi: 10.1038/s41698-017-0040-z.
Calder PC. n-3 fatty acids, inflammation and immunity: new mechanisms to explain old actions. Proc Nutr Soc. 2013 Aug;72(3):326-36. doi: 10.1017/S0029665113001031.
Calder PC. Polyunsaturated fatty acids and inflammatory processes: New twists in an old tale. Biochimie. 2009 Jun;91(6):791-5. doi: 10.1016/j.biochi.2009.01.008.
Chajès V, Sattler W, Stranzl A, Kostner GM. Influence of n-3 fatty acids on the growth of human breast cancer cells in vitro: relationship to peroxides and vitamin-E. Breast Cancer Res Treat. 1995 Jun;34(3):199-212. doi: 10.1007/BF00689711.
Chamberland JP, Moon HS. Down-regulation of malignant potential by alpha linolenic acid in human and mouse colon cancer cells9. Fam Cancer. 2015 Mar;14(1):25-30. doi: 10.1007/s10689-014-9762-z.
Chen LH, Hu Q, Li G, Zhang L, Qin LQ, Zuo H, Xu G. Dietary Intake and Biomarkers of α-Linolenic Acid and Mortality: A Meta-Analysis of Prospective Cohort Studies. Front Nutr. 2021 Nov 3;8:743852. doi: 10.3389/fnut.2021.743852.
Christensen JH, Fabrin K, Borup K, Barber N, Poulsen J. Prostate tissue and leukocyte levels of n-3 polyunsaturated fatty acids in men with benign prostate hyperplasia or prostate cancer. BJU Int. 2006 Feb;97(2):270-3. doi: 10.1111/j.1464-410X.2006.05951.x.
Davidson MH. Omega-3 fatty acids: new insights into the pharmacology and biology of docosahexaenoic acid, docosapentaenoic acid, and eicosapentaenoic acid. Curr Opin Lipidol. 2013 Dec;24(6):467-74. doi: 10.1097/MOL.0000000000000019.
de Goede J, Verschuren WM, Boer JM, Kromhout D, Geleijnse JM. Alpha-linolenic acid intake and 10-year incidence of coronary heart disease and stroke in 20,000 middle-aged men and women in the Netherlands. PLoS One. 2011 Mar 25;6(3):e17967. doi: 10.1371/journal.pone.0017967.
de Lorgeril M, Salen P. The Mediterranean diet in secondary prevention of coronary heart disease. Clin Invest Med. 2006 Jun;29(3):154-8.
Del Gobbo LC, Imamura F, Aslibekyan S, Marklund M, Virtanen JK, Wennberg M, Yakoob MY, Chiuve SE, Dela Cruz L, Frazier-Wood AC, Fretts AM, Guallar E, Matsumoto C, Prem K, Tanaka T, Wu JH, Zhou X, Helmer C, Ingelsson E, Yuan JM, Barberger-Gateau P, Campos H, Chaves PH, Djoussé L, Giles GG, Gómez-Aracena J, Hodge AM, Hu FB, Jansson JH, Johansson I, Khaw KT, Koh WP, Lemaitre RN, Lind L, Luben RN, Rimm EB, Risérus U, Samieri C, Franks PW, Siscovick DS, Stampfer M, Steffen LM, Steffen BT, Tsai MY, van Dam RM, Voutilainen S, Willett WC, Woodward M, Mozaffarian D; Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCe). ω-3 Polyunsaturated Fatty Acid Biomarkers and Coronary Heart Disease: Pooling Project of 19 Cohort Studies. JAMA Intern Med. 2016 Aug 1;176(8):1155-66. doi: 10.1001/jamainternmed.2016.2925.
Djoussé L, Arnett DK, Pankow JS, Hopkins PN, Province MA, Ellison RC. Dietary linolenic acid is associated with a lower prevalence of hypertension in the NHLBI Family Heart Study. Hypertension. 2005 Mar;45(3):368-73. doi: 10.1161/01.HYP.0000154679.41568.e6.
Djoussé L, Folsom AR, Province MA, Hunt SC, Ellison RC; National Heart, Lung, and Blood Institute Family Heart Study. Dietary linolenic acid and carotid atherosclerosis: the National Heart, Lung, and Blood Institute Family Heart Study. Am J Clin Nutr. 2003 Apr;77(4):819-25. doi: 10.1093/ajcn/77.4.819.
Djoussé L, Hunt SC, Arnett DK, Province MA, Eckfeldt JH, Ellison RC. Dietary linolenic acid is inversely associated with plasma triacylglycerol: the National Heart, Lung, and Blood Institute Family Heart Study. Am J Clin Nutr. 2003 Dec;78(6):1098-102. doi: 10.1093/ajcn/78.6.1098.
Djoussé L, Rautaharju PM, Hopkins PN, Whitsel EA, Arnett DK, Eckfeldt JH, Province MA, Ellison RC; Investigators of the NHLBI Family Heart Study. Dietary linolenic acid and adjusted QT and JT intervals in the National Heart, Lung, and Blood Institute Family Heart study. J Am Coll Cardiol. 2005 May 17;45(10):1716-22. doi: 10.1016/j.jacc.2005.01.060.
Dugani A, Auzzi A, Naas F, Megwez S. Effects of the oil and mucilage from flaxseed (linum usitatissimum) on gastric lesions induced by ethanol in rats. Libyan J Med. 2008 Dec 1;3(4):166-9. doi: 10.4176/080612.
Egert S, Kannenberg F, Somoza V, Erbersdobler HF, Wahrburg U. Dietary alpha-linolenic acid, EPA, and DHA have differential effects on LDL fatty acid composition but similar effects on serum lipid profiles in normolipidemic humans. J Nutr. 2009 May;139(5):861-8. doi: 10.3945/jn.108.103861.
Hanson S, Thorpe G, Winstanley L, Abdelhamid AS, Hooper L; PUFAH group. Omega-3, omega-6 and total dietary polyunsaturated fat on cancer incidence: systematic review and meta-analysis of randomised trials. Br J Cancer. 2020 Apr;122(8):1260-1270. doi: 10.1038/s41416-020-0761-6.
Harris WS, Sands SA, Windsor SL, Ali HA, Stevens TL, Magalski A, Porter CB, Borkon AM. Omega-3 fatty acids in cardiac biopsies from heart transplantation patients: correlation with erythrocytes and response to supplementation. Circulation. 2004 Sep 21;110(12):1645-9. doi: 10.1161/01.CIR.0000142292.
Harris WS, Tintle NL, Imamura F, Qian F, Korat AVA, Marklund M, Djoussé L, Bassett JK, Carmichael PH, Chen YY, Hirakawa Y, Küpers LK, Laguzzi F, Lankinen M, Murphy RA, Samieri C, Senn MK, Shi P, Virtanen JK, Brouwer IA, Chien KL, Eiriksdottir G, Forouhi NG, Geleijnse JM, Giles GG, Gudnason V, Helmer C, Hodge A, Jackson R, Khaw KT, Laakso M, Lai H, Laurin D, Leander K, Lindsay J, Micha R, Mursu J, Ninomiya T, Post W, Psaty BM, Risérus U, Robinson JG, Shadyab AH, Snetselaar L, Sala-Vila A, Sun Y, Steffen LM, Tsai MY, Wareham NJ, Wood AC, Wu JHY, Hu F, Sun Q, Siscovick DS, Lemaitre RN, Mozaffarian D; Fatty Acids and Outcomes Research Consortium (FORCE). Blood n-3 fatty acid levels and total and cause-specific mortality from 17 prospective studies. Nat Commun. 2021 Apr 22;12(1):2329. doi: 10.1038/s41467-021-22370-2.
Hsiao WT, Su HM, Su KP, Chen SH, Wu HP, You YL, Fu RH, Chao PM. Deficiency or activation of peroxisome proliferator-activated receptor α reduces the tissue concentrations of endogenously synthesized docosahexaenoic acid in C57BL/6J mice. Nutr Res Pract. 2019 Aug;13(4):286-294. doi: 10.4162/nrp.2019.13.4.286.
Jelinek GA, Hadgkiss EJ, Weiland TJ, Pereira NG, Marck CH, van der Meer DM. Association of fish consumption and Ω 3 supplementation with quality of life, disability and disease activity in an international cohort of people with multiple sclerosis. Int J Neurosci. 2013 Nov;123(11):792-800. doi: 10.3109/00207454.2013.803104.
Jeppesen PB, Høy CE, Mortensen PB. Deficiencies of essential fatty acids, vitamin A and E and changes in plasma lipoproteins in patients with reduced fat absorption or intestinal failure. Eur J Clin Nutr. 2000 Aug;54(8):632-42. doi: 10.1038/sj.ejcn.1601067.
Jump DB, Tripathy S, Depner CM. Fatty acid-regulated transcription factors in the liver. Annu Rev Nutr. 2013;33:249-69. doi: 10.1146/annurev-nutr-071812-161139.
Kaithwas G, Majumdar DK. Therapeutic effect of Linum usitatissimum (flaxseed/linseed) fixed oil on acute and chronic arthritic models in albino rats. Inflammopharmacology. 2010 Jun;18(3):127-36. doi: 10.1007/s10787-010-0033-9.
Kitson AP, Stroud CK, Stark KD. Elevated production of docosahexaenoic acid in females: potential molecular mechanisms. Lipids. 2010 Mar;45(3):209-24. doi: 10.1007/s11745-010-3391-6.
Klein V, Chajès V, Germain E, Schulgen G, Pinault M, Malvy D, Lefrancq T, Fignon A, Le Floch O, Lhuillery C, Bougnoux P. Low alpha-linolenic acid content of adipose breast tissue is associated with an increased risk of breast cancer. Eur J Cancer. 2000 Feb;36(3):335-40. doi: 10.1016/s0959-8049(99)00254-3.
Krauss RM, Eckel RH, Howard B, Appel LJ, Daniels SR, Deckelbaum RJ, Erdman JW Jr, Kris-Etherton P, Goldberg IJ, Kotchen TA, Lichtenstein AH, Mitch WE, Mullis R, Robinson K, Wylie-Rosett J, St Jeor S, Suttie J, Tribble DL, Bazzarre TL. AHA Dietary Guidelines: revision 2000: A statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation. 2000 Oct 31;102(18):2284-99. doi: 10.1161/01.cir.102.18.2284.
Lee TC, Ivester P, Hester AG, Sergeant S, Case LD, Morgan T, Kouba EO, Chilton FH. The impact of polyunsaturated fatty acid-based dietary supplements on disease biomarkers in a metabolic syndrome/diabetes population. Lipids Health Dis. 2014 Dec 16;13:196. doi: 10.1186/1476-511X-13-196.
Liu J, Ma DW. The role of n-3 polyunsaturated fatty acids in the prevention and treatment of breast cancer. Nutrients. 2014 Nov 18;6(11):5184-223. doi: 10.3390/nu6115184.
Lucas M, Mirzaei F, O’Reilly EJ, Pan A, Willett WC, Kawachi I, Koenen K, Ascherio A. Dietary intake of n-3 and n-6 fatty acids and the risk of clinical depression in women: a 10-y prospective follow-up study. Am J Clin Nutr. 2011 Jun;93(6):1337-43. doi: 10.3945/ajcn.111.011817.
Mathias RA, Pani V, Chilton FH. Genetic Variants in the FADS Gene: Implications for Dietary Recommendations for Fatty Acid Intake. Curr Nutr Rep. 2014 Jun;3(2):139-148. doi: 10.1007/s13668-014-0079-1.
McNamara RK, Almeida DM. Omega-3 Polyunsaturated Fatty Acid Deficiency and Progressive Neuropathology in Psychiatric Disorders: A Review of Translational Evidence and Candidate Mechanisms. Harv Rev Psychiatry. 2019 Mar/Apr;27(2):94-107. doi: 10.1097/HRP.0000000000000199.
Merchant AT, Curhan GC, Rimm EB, Willett WC, Fawzi WW. Intake of n-6 and n-3 fatty acids and fish and risk of community-acquired pneumonia in US men. Am J Clin Nutr. 2005 Sep;82(3):668-74. doi: 10.1093/ajcn.82.3.668.
Miura K, Way M, Jiyad Z, Marquart L, Plasmeijer EI, Campbell S, Isbel N, Fawcett J, Ferguson LE, Davis M, Whiteman DC, Soyer HP, O’Rourke P, Green AC. Omega-3 fatty acid intake and decreased risk of skin cancer in organ transplant recipients. Eur J Nutr. 2021 Jun;60(4):1897-1905. doi: 10.1007/s00394-020-02378-y.
Moon HS, Batirel S, Mantzoros CS. Alpha linolenic acid and oleic acid additively down-regulate malignant potential and positively cross-regulate AMPK/S6 axis in OE19 and OE33 esophageal cancer cells. Metabolism. 2014 Nov;63(11):1447-54. doi: 10.1016/j.metabol.2014.07.009.
Naghshi S, Aune D, Beyene J, Mobarak S, Asadi M, Sadeghi O. Dietary intake and biomarkers of alpha linolenic acid and risk of all cause, cardiovascular, and cancer mortality: systematic review and dose-response meta-analysis of cohort studies. BMJ. 2021 Oct 13;375:n2213. doi: 10.1136/bmj.n2213.
Patterson E, Wall R, Fitzgerald GF, Ross RP, Stanton C. Health implications of high dietary omega-6 polyunsaturated Fatty acids. J Nutr Metab. 2012;2012:539426. doi: 10.1155/2012/539426.
Rajaram S. Health benefits of plant-derived α-linolenic acid. Am J Clin Nutr. 2014 Jul;100 Suppl 1:443S-8S. doi: 10.3945/ajcn.113.071514.
Saito S, Fukuhara I, Osaki N, Nakamura H, Katsuragi Y. Consumption of alpha-Linolenic Acid-enriched Diacylglycerol Reduces Visceral Fat Area in Overweight and Obese Subjects: a Randomized, Double-blind Controlled, Parallel-group Designed Trial. J Oleo Sci. 2016 Jul 1;65(7):603-11. doi: 10.5650/jos.ess16059.
Simopoulos AP. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients. 2016 Mar 2;8(3):128. doi: 10.3390/nu8030128.
Soleimani Z, Hashemdokht F, Bahmani F, Taghizadeh M, Memarzadeh MR, Asemi Z. Clinical and metabolic response to flaxseed oil omega-3 fatty acids supplementation in patients with diabetic foot ulcer: A randomized, double-blind, placebo-controlled trial. J Diabetes Complications. 2017 Sep;31(9):1394-1400. doi: 10.1016/j.jdiacomp.2017.06.010.
Stark AH, Reifen R, Crawford MA. Past and Present Insights on Alpha-linolenic Acid and the Omega-3 Fatty Acid Family. Crit Rev Food Sci Nutr. 2016 Oct 25;56(14):2261-7. doi: 10.1080/10408398.2013.828678.
Stegink LD, Freeman JB, Wispe J, Connor WE. Absence of the biochemical symptoms of essential fatty acid deficiency in surgical patients undergoing protein sparing therapy. Am J Clin Nutr. 1977 Mar;30(3):388-93. doi: 10.1093/ajcn/30.3.388.
Stillwell W, Wassall SR. Docosahexaenoic acid: membrane properties of a unique fatty acid. Chem Phys Lipids. 2003 Nov;126(1):1-27. doi: 10.1016/s0009-3084(03)00101-4.
Taylor KL, Hadgkiss EJ, Jelinek GA, Weiland TJ, Pereira NG, Marck CH, van der Meer DM. Lifestyle factors, demographics and medications associated with depression risk in an international sample of people with multiple sclerosis. BMC Psychiatry. 2014 Dec 3;14:327. doi: 10.1186/s12888-014-0327-3.
Thompson L, Cockayne A, Spiller RC. Inhibitory effect of polyunsaturated fatty acids on the growth of Helicobacter pylori: a possible explanation of the effect of diet on peptic ulceration. Gut. 1994 Nov;35(11):1557-61. doi: 10.1136/gut.35.11.1557.
Thompson LU, Chen JM, Li T, Strasser-Weippl K, Goss PE. Dietary flaxseed alters tumor biological markers in postmenopausal breast cancer. Clin Cancer Res. 2005 May 15;11(10):3828-35. doi: 10.1158/1078-0432.CCR-04-2326.
Tosi F, Sartori F, Guarini P, Olivieri O, Martinelli N. Delta-5 and delta-6 desaturases: crucial enzymes in polyunsaturated fatty acid-related pathways with pleiotropic influences in health and disease. Adv Exp Med Biol. 2014;824:61-81. doi: 10.1007/978-3-319-07320-0_7.
Truan JS, Chen JM, Thompson LU. Flaxseed oil reduces the growth of human breast tumors (MCF-7) at high levels of circulating estrogen. Mol Nutr Food Res. 2010 Oct;54(10):1414-21. doi: 10.1002/mnfr.200900521.
Wei J, Hou R, Xi Y, Kowalski A, Wang T, Yu Z, Hu Y, Chandrasekar EK, Sun H, Ali MK. The association and dose-response relationship between dietary intake of α-linolenic acid and risk of CHD: a systematic review and meta-analysis of cohort studies. Br J Nutr. 2018 Jan;119(1):83-89. doi: 10.1017/S0007114517003294.
Weiland TJ, Jelinek GA, Marck CH, Hadgkiss EJ, van der Meer DM, Pereira NG, Taylor KL. Clinically significant fatigue: prevalence and associated factors in an international sample of adults with multiple sclerosis recruited via the internet. PLoS One. 2015 Feb 18;10(2):e0115541. doi: 10.1371/journal.pone.0115541.
Wilkinson P, Leach C, Ah-Sing EE, Hussain N, Miller GJ, Millward DJ, Griffin BA. Influence of alpha-linolenic acid and fish-oil on markers of cardiovascular risk in subjects with an atherogenic lipoprotein phenotype. Atherosclerosis. 2005 Jul;181(1):115-24. doi: 10.1016/j.atherosclerosis.2004.12.029.
Zuliani G, Galvani M, Leitersdorf E, Volpato S, Cavalieri M, Fellin R. The role of polyunsaturated fatty acids (PUFA) in the treatment of dyslipidemias. Curr Pharm Des. 2009;15(36):4087-93. doi: 10.2174/138161209789909773.

Merch and Household Goods
Check out our collection of practical and nerdtastic T-shirts, totes, and more!